Characterization of zolbetuximab in pancreatic cancer models
- PMID: 30546962
- PMCID: PMC6287799
- DOI: 10.1080/2162402X.2018.1523096
Characterization of zolbetuximab in pancreatic cancer models
Abstract
In healthy tissue, the tight junction protein Claudin 18.2 (CLDN18.2) is present only in the gastric mucosa. Upon malignant transformation of gastric epithelial tissue, perturbations in cell polarity lead to cell surface exposure of CLDN18.2 epitopes. Moreover, CLDN18.2 is aberrantly expressed in malignancies of several other organs, such as pancreatic cancer (PC). A monoclonal antibody, zolbetuximab (formerly known as IMAB362), has been generated against CLDN18.2. In a phase 2 clinical trial (FAST: NCT01630083), zolbetuximab in conjunction with chemotherapy prolonged overall and progression-free survival over chemotherapy alone and improved quality of life. In this study, the mechanism of action and antitumor activity of zolbetuximab were investigated using nonclinical PC models. Zolbetuximab bound specifically and with strong affinity to human PC cells that expressed CLDN18.2 on the cell surface. In ex vivo systems using immune effector cells and serum from healthy donors, zolbetuximab induced antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), resulting in the lysis of cultured human PC cells. The amplitude of ADCC and CDC directly correlated with cell surface CLDN18.2 levels. The chemotherapeutic agent gemcitabine upregulated CLDN18.2 expression in cultured human PC cells and enhanced zolbetuximab-induced ADCC. In mouse xenograft tumors derived from human PC cell lines, including gemcitabine-refractory ones, zolbetuximab slowed tumor growth, benefited survival, and attenuated metastases development. The results presented here validate CLDN18.2 as a targetable biomarker in PC and support extension of the clinical development of zolbetuximab to patients with CLDN18.2-expressing PC.
Keywords: ADCC; Claudin 18.2; IMAB362; antibody-dependent cellular cytotoxicity; complement-dependent cytotoxicity; immunotherapy; monoclonal antibody; pancreatic cancer; targeted therapy; zolbetuximab.
Figures

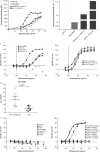
(a) Typical image of CLDN18.2+ pancreatic ductal adenocarcinoma. Staining was performed with 43-14A antibody. Magnification: 200x.
(b) Transcript (qRT-PCR) and (b) protein (western blot) levels of CLDN18.2 in PC cell lines with endogenous (light red bars) and transduced (dark red bars) CLDN18.2 expression. qRT-PCR data are mean ± SD of 1–9 independent measurements. Cell lines with a relative expression level above 1 × 105 were considered CLDN18.2+ (dotted line in a). Gray bars represent non-PC cell lines and controls.
(c) Detection of CLDN18 protein in pancreatic cancer cell lysates. Western blot analysis was performed using a CLDN18 antibody detecting the C-terminal of CLDN18.1 and CLDN18.2 (C-term, Zymed) and a loading control antibody detecting β-actin. Lysates of SKBR-3 cells were used as negative control, whereas lysates of HEK293 cells stably transfected with CLDN18.2 (HEK293-p740) were used as positive control. BxPC-3 (e) and BxPC-3 (a) represent BxPC-3 cell lines from ECACC and ATCC, respectively.
(d) Effect of treatment with Gem or GemOx on CLDN18.2 mRNA expression. mRNA was isolated from DAN-G cells (untreated, treated with Gem [1 ng/mL] or GemOx [Gem 1 ng/mL + Ox 10 ng/mL] for 2 days) or Patu 8988S cells (untreated or treated with Gem [10 ng/mL] or GemOx [Gem 10 ng/mL + Ox 100 ng/mL] for 3 days). RNA was reverse transcribed to cDNA and CLDN18.2 transcript levels analyzed by qRT-PCR. Expression levels are depicted relative to the housekeeping gene HPRT.
(e) Effect of treatment with Gem or GemOx on CLDN18 protein expression. CLDN18 protein expression was analyzed in total cell lysates of untreated, Gem- (1 ng/mL) or GemOx- (Gem 10 ng/mL + Ox 100 ng/mL) treated DAN-G, or Patu 8988S cells by Western blot and detected with the Zymed C-term polyclonal antibody. Actin served as loading control.
(f) Influence of Gem, and 5-FU on surface expression of CLDN18.2 in Patu 8988S pancreatic cancer cells. CLDN18.2 expression was detected using flow cytometry with zolbetuximab as primary antibody and anti-hu-IgG-APC as secondary antibody. Histogram shows CLDN18.2 expression on Patu 8988S cells treated for 3 days with DMSO (gray line), 10 ng/mL Gem, 500 ng/mL 5-FU, 100 ng/mL PTX, or 500 ng/mL Ox (red line).


Similar articles
-
FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma.Ann Oncol. 2021 May;32(5):609-619. doi: 10.1016/j.annonc.2021.02.005. Epub 2021 Feb 19. Ann Oncol. 2021. PMID: 33610734 Clinical Trial.
-
Zolbetuximab for Unresectable and Metastatic Gastric and Gastroesophageal Junction Adenocarcinoma: A Review of Literature.Cureus. 2024 Dec 6;16(12):e75206. doi: 10.7759/cureus.75206. eCollection 2024 Dec. Cureus. 2024. PMID: 39759684 Free PMC article. Review.
-
A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study.Ann Oncol. 2019 Sep 1;30(9):1487-1495. doi: 10.1093/annonc/mdz199. Ann Oncol. 2019. PMID: 31240302 Free PMC article. Clinical Trial.
-
Zolbetuximab for Claudin18.2-positive gastric or gastroesophageal junction cancer.Ther Adv Med Oncol. 2024 Jan 3;16:17588359231217967. doi: 10.1177/17588359231217967. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 38188462 Free PMC article. Review.
-
Effect of anti-claudin 18.2 monoclonal antibody zolbetuximab alone or combined with chemotherapy or programmed cell death-1 blockade in syngeneic and xenograft gastric cancer models.J Pharmacol Sci. 2024 Jul;155(3):84-93. doi: 10.1016/j.jphs.2024.04.004. Epub 2024 Apr 18. J Pharmacol Sci. 2024. PMID: 38797537
Cited by
-
Targeting CLDN18.2 in cancers of the gastrointestinal tract: New drugs and new indications.Front Oncol. 2023 Mar 10;13:1132319. doi: 10.3389/fonc.2023.1132319. eCollection 2023. Front Oncol. 2023. PMID: 36969060 Free PMC article. Review.
-
Dark horse target Claudin18.2 opens new battlefield for pancreatic cancer.Front Oncol. 2024 Mar 6;14:1371421. doi: 10.3389/fonc.2024.1371421. eCollection 2024. Front Oncol. 2024. PMID: 38511141 Free PMC article. Review.
-
Multiplex immunohistochemistry defines the tumor immune microenvironment and immunotherapeutic outcome in CLDN18.2-positive gastric cancer.BMC Med. 2022 Jul 11;20(1):223. doi: 10.1186/s12916-022-02421-1. BMC Med. 2022. PMID: 35811317 Free PMC article.
-
Pancreas cancer: Therapeutic trials in metastatic disease.J Surg Oncol. 2021 May;123(6):1475-1488. doi: 10.1002/jso.26359. J Surg Oncol. 2021. PMID: 33831245 Free PMC article. Review.
-
Phase 1 trial of zolbetuximab in Japanese patients with CLDN18.2+ gastric or gastroesophageal junction adenocarcinoma.Cancer Sci. 2023 Apr;114(4):1606-1615. doi: 10.1111/cas.15684. Epub 2022 Dec 25. Cancer Sci. 2023. PMID: 36478334 Free PMC article. Clinical Trial.
References
-
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, Oomen LA, Peipp M, Valerius T, Slootstra JW, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. doi:10.4049/jimmunol.1003032. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
