Therapeutic vaccination using minimal HPV16 epitopes in a novel MHC-humanized murine HPV tumor model
- PMID: 30546964
- PMCID: PMC6287800
- DOI: 10.1080/2162402X.2018.1524694
Therapeutic vaccination using minimal HPV16 epitopes in a novel MHC-humanized murine HPV tumor model
Abstract
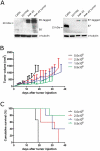
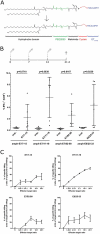
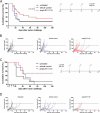
Therapeutic vaccination as a treatment option for HPV-induced cancers is actively pursued because the two HPV proteins E6 and E7 represent ideal targets for immunotherapy, as they are non-self and expressed in all tumor stages. MHC-humanized mice are valuable tools for the study of therapeutic cancer vaccines - given the availability of a suitable tumor model. Here, we present for the first time an HPV16 tumor model suitable for fully MHC-humanized A2.DR1 mice, PAP-A2 cells, which in contrast to existing HPV16 tumor models allows the exclusive study of HLA-A2- and DR1-mediated immune responses, without any interfering murine MHC-presented epitopes. We used several HPV16 epitopes that were shown to be presented on human cervical cancer cells by mass spectrometry for therapeutic anti-tumor vaccination in the new tumor model. All epitopes were immunogenic when rendered amphiphilic by incorporation into a molecule containing stearic acids. Prophylactic and therapeutic vaccination experiments with the epitope E7/11-19 demonstrated that effective immune responses could be induced with these vaccination approaches in A2.DR1 mice. Interestingly, the combination of E7/11-19 with other immunogenic HPV16 E6/E7 epitopes caused a reduction of vaccine efficacy, although all tested combinations resulted in a survival benefit. In summary, we present the first HPV16 tumor model for exclusive studies of HLA-A2-mediated anti-HPV tumor immune responses and show anti-tumor efficacy of minimal epitope vaccines.
Keywords: A2.DR1; Cancer immunotherapy; HLA-humanized mouse model; PAP-A2; human papillomavirus (HPV); therapeutic vaccination.
Figures



References
-
- Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, Porras C, Schiffman M, Sidawy M, Schiller JT, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2015;215:212.e1–212.e15. - PMC - PubMed
-
- Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — united States, 2016. MMWR Morb Mortal Wkly Rep. 2016;66:874–882. doi: 10.15585/mmwr.mm6633a2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
