Clinical characterization of colitis arising from anti-PD-1 based therapy
- PMID: 30546965
- PMCID: PMC6287774
- DOI: 10.1080/2162402X.2018.1524695
Clinical characterization of colitis arising from anti-PD-1 based therapy
Abstract
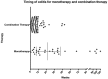
Colitis is a frequent, clinically-significant immune-related adverse event caused by anti-programmed death-1 (PD-1). The clinical features, timing, and management of colitis with anti-PD-1-based regimens are not well-characterized. Patients with advanced melanoma that received either anti-PD-1 monotherapy ("monotherapy") or combined with ipilimumab ("combination therapy") were screened from 8 academic medical centers, to identify those with clinically-relevant colitis (colitis requiring systemic steroids). Of 1261 patients who received anti-PD-1-based therapy, 109 experienced colitis. The incidence was 3.2% (30/937) and 24.4% (79/324) in the monotherapy and combination therapy cohorts, respectively. Patients with colitis from combination therapy had significantly earlier symptom onset (7.2 weeks vs 25.4 weeks, p < 0.0001), received higher steroid doses (median prednisone equivalent 1.5 mg/kg vs 1.0 mg/kg, p = 0.0015) and experienced longer steroid tapers (median 6.0 vs 4.0 weeks, p = 0.0065) compared to monotherapy. Infliximab use and steroid-dose escalation occurred more frequently in the combination therapy cohort compared to monotherapy. Nearly all patients had resolution of their symptoms although one patient died from complications. Anti-PD-1 associated colitis has a variable clinical presentation, and is more frequent and severe when associated with combination therapy. This variability in checkpoint-inhibitor associated colitis suggests that further optimization of treatment algorithms is needed.
Keywords: Colitis; anti-programmed-death-1; immune-related adverse events; immunotherapy; melanoma.
Figures



References
-
- Hodi FS, Kluger H, Sznol M, Carvajal RD, Lawrence D, Atkins MB, Powderly JD, Sharfman WH, Puzanov I, Smith D, et al. Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. In In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research New Orleans, LA: Philadelphia (PA): AACR; 2016. 10.1158/1538-7445.AM2016-CT001 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
