Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer's disease
- PMID: 30547227
- PMCID: PMC6514201
- DOI: 10.1007/s00401-018-1948-2
Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer's disease
Abstract
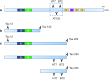
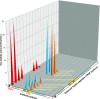
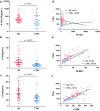
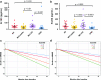
Tau is an axonal microtubule-binding protein. Tau pathology in brain and increased tau concentration in the cerebrospinal fluid (CSF) are hallmarks of Alzheimer's disease (AD). Most of tau in CSF is present as fragments. We immunoprecipitated tau from CSF and identified several endogenous peptides ending at amino acid (aa) 123 or 224 using high-resolution mass spectrometry. We raised neo-epitope-specific antibodies against tau fragments specifically ending at aa 123 and 224, respectively. With these antibodies, we performed immunohistochemistry on brain tissue and designed immunoassays measuring N-123, N-224, and x-224 tau. Immunoassays were applied to soluble brain fractions from pathologically confirmed subjects (81 AD patients, 33 controls), CSF from three cross-sectional and two longitudinal cohorts (a total of 133 AD, 38 MCI, 20 MCI-AD, 31 PSP, 15 CBS patients, and 91 controls), and neuronally- and peripherally-derived extracellular vesicles (NDEVs and PDEVs, respectively) in serum from four AD patients and four controls. Anti-tau 224 antibody stained neurofibrillary tangles and neuropil threads, while anti-tau 123 only showed weak cytoplasmic staining in AD. N-224 tau was lower in the AD soluble brain fraction compared to controls, while N-123 tau showed similar levels. N-224 tau was higher in AD compared to controls in all CSF cohorts (p < 0.001), but not N-123 tau. Decrease in cognitive performance and conversion from MCI to AD were associated with increased baseline CSF levels of N-224 tau (p < 0.0001). N-224 tau concentrations in PSP and CBS were significantly lower than in AD (p < 0.0001) and did not correlate to t-tau and p-tau. In a longitudinal cohort, CSF N-224 tau levels were stable over 6 months, with no significant effect of treatment with AChE inhibitors. N-224 tau was present in NDEVs, while N-123 tau showed comparable concentrations in both vesicle types. We suggest that N-123 tau is produced both in CNS and PNS and represents a general marker of tau metabolism, while N-224 tau is neuron-specific, present in the tangles, secreted in CSF, and upregulated in AD, suggesting a link between tau cleavage and propagation, tangle pathology, and cognitive decline.
Keywords: Alzheimer’s disease; Cerebrospinal fluid; Immunohistochemistry; Mass spectrometry; Tau fragments.
Conflict of interest statement
OH has acquired research support (for the institution) from Roche, GE Healthcare, Biogen, AVID Radiopharmaceuticals, Fujirebio, and Euroimmun. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, Roche, and Fujirebio. KW and HHC are employed at Genentech, South San Francisco, CA, USA. HZ has served at scientific advisory board meetings for Roche Diagnostics, Wave, Samumed, and CogRx, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. LP has served at scientific advisory board meetings for Fujirebio, IBL and Roche.
Figures





References
-
- Amadoro G, Corsetti V, Atlante A, Florenzano F, Capsoni S, Bussani R, et al. Interaction between NH(2)-tau fragment and Abeta in Alzheimer’s disease mitochondria contributes to the synaptic deterioration. Neurobiol Aging. 2012;33(833):833-e1. doi: 10.1016/j.neurobiolaging.2011.08.001. - DOI - PubMed
-
- Amadoro G, Corsetti V, Sancesario GM, Lubrano A, Melchiorri G, Bernardini S, et al. Cerebrospinal fluid levels of a 20–22 kDa NH2 fragment of human tau provide a novel neuronal injury biomarker in Alzheimer’s disease and other dementias. J Alzheimer’s Dis. 2014;42:211–226. doi: 10.3233/JAD-140267. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

