Levodopa-carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry
- PMID: 30547712
- PMCID: PMC6360350
- DOI: 10.2217/nmt-2018-0034
Levodopa-carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry
Abstract
Aim: To evaluate long-term effects of levodopa-carbidopa intestinal gel on dyskinesia burden.
Patients & methods: Posthoc analysis of the GLORIA registry assessed subgroups of advanced Parkinson's disease patients with <4 and ≥4 h/day of levodopa-induced dyskinesia at baseline.
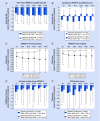
Results & conclusions: Mean dyskinesia duration significantly (p < 0.0001) decreased by 3.5 h in patients with ≥4 h baseline dyskinesia; conversely, dyskinesia duration increased by 1.6 h in patients with <4 h baseline dyskinesia. Quality of life improved in both subgroups. Adverse drug reactions occurred at similar rates in both subgroups. Despite increases in levodopa dose, levodopa-carbidopa intestinal gel treatment led to significant and sustained reductions in dyskinesia time, severity and associated pain in advanced Parkinson's disease patients with high baseline dyskinesia burden.
Keywords: Parkinson’s disease; dyskinesia; levodopa; quality of life.
Conflict of interest statement
This study was supported by AbbVie Inc., North Chicago, IL, USA. AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing and approving the manuscript for publication. W Poewe was a study investigator and has received compensation from AbbVie, Astra Zeneca, Teva, Novartis, BIAL, Biogen, Britannia, Neuroderm, UCB, Orion Pharma, Takeda, Roche, Zambon and Merz Pharmaceuticals (for consultancy and lecture fees in relation to clinical drug development programs for Parkinson's disease) outside the submitted work. He has also received royalties from Thieme, Wiley-Blackwell and Oxford University Press. KR Chaudhuri was a study investigator and has received honorarium from UCB, AbbVie, Britannia, Mundipharma, Boehringer Ingelheim and GSK Pharmaceuticals for lecturing at symposia. He has acted as a consultant for UCB, AbbVie, Britannia, Neuronova and Mundipharma. KRC acknowledges independent research presented in this paper partly funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. He has also received research funding from Parkinson's UK, PDNMG, EU Horizon 2020 and IMI initiative, as well as educational grants from UCB, Britannia, AbbVie, GSK Pharmaceuticals, Boehringer Ingelheim and Neuronova. In addition, he has received royalties from Oxford University Press and holds intellectual property rights for the Kings Parkinson's Pain Scale and Parkinson's Disease Sleep Scale 2. L Bergmann is an employee of AbbVie and owns stock or stock options. A Antonini was a study investigator and has received compensation for consultancy and speaker-related activities from UCB, Boston Scientific, Boehringer Ingelheim, AbbVie and Zambon. He has received research support from Mundipharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support, funded by AbbVie, was provided by KM Cameron of JB Ashtin, who developed the first draft based on an author-approved outline and assisted in implementing author revisions.
Figures
References
-
- Mouradian MM, Heuser IJ, Baronti F, Fabbrini G, Juncos JL, Chase TN. Pathogenesis of dyskinesias in Parkinson's disease. Ann. Neurol. 1989;25(5):523–526. - PubMed
-
- Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson's disease. Trends Neurosci. 2000;23(10 Suppl.):S2–S7. - PubMed
-
- Antonini A, Moro E, Godeiro C, Reichmann H. Medical and surgical management of advanced Parkinson's disease. Mov. Disord. 2018;33(6):900–908. - PubMed
-
- Chaudhuri KR, Poewe W, Brooks DJ. Motor and nonmotor complications of levodopa: phenomenology, risk factors, and imaging features. Mov. Disord. 2018;33(6):909–919. In press. - PubMed
-
- Nutt JG. Pharmacokinetics and pharmacodynamics of levodopa. Mov. Disord. 2008;23(Suppl. 3):S580–S584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
