Using automated electronic medical record data extraction to model ALS survival and progression
- PMID: 30547800
- PMCID: PMC6295028
- DOI: 10.1186/s12883-018-1208-z
Using automated electronic medical record data extraction to model ALS survival and progression
Abstract
Background: To assess the feasibility of using automated capture of Electronic Medical Record (EMR) data to build predictive models for amyotrophic lateral sclerosis (ALS) outcomes.
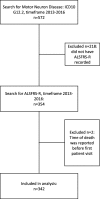
Methods: We used an Informatics for Integrating Biology and the Bedside search discovery tool to identify and extract data from 354 ALS patients from the University of Kansas Medical Center EMR. The completeness and integrity of the data extraction were verified by manual chart review. A linear mixed model was used to model disease progression. Cox proportional hazards models were used to investigate the effects of BMI, gender, and age on survival.
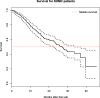
Results: Data extracted from the EMR was sufficient to create simple models of disease progression and survival. Several key variables of interest were unavailable without including a manual chart review. The average ALS Functional Rating Scale - Revised (ALSFRS-R) baseline score at first clinical visit was 34.08, and average decline was - 0.64 per month. Median survival was 27 months after first visit. Higher baseline ALSFRS-R score and BMI were associated with improved survival, higher baseline age was associated with decreased survival.
Conclusions: This study serves to show that EMR-captured data can be extracted and used to track outcomes in an ALS clinic setting, potentially important for post-marketing research of new drugs, or as historical controls for future studies. However, as automated EMR-based data extraction becomes more widely used there will be a need to standardize ALS data elements and clinical forms for data capture so data can be pooled across academic centers.
Keywords: Amyotrophic lateral sclerosis; Disease progression; Electronic medical record; Motor neuron disease.
Conflict of interest statement
Ethics approval and consent to participate
This retrospective chart review was IRB-approved and received a waiver of consent by University of Kansas Medical Center Human Research Protection Program Institutional Review Board (IRB# STUDY00004291). Patient data was managed on secure servers at KUMC.
Consent for publication
Not applicable
Competing interests
JS is a consultant for aTyr, Acceleron, Fulcrum, Regeneron, and Strongbridge. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- WRITING GROUP ON BEHALF OF THE EDARAVONE (MCI-186) ALS 18 STUDY GROUP Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: grade 3, requiring assistance for eating, excretion or ambulation) Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(sup1):40–48. doi: 10.1080/21678421.2017.1361441. - DOI - PubMed
-
- Katyal N, Govindarajan R. Shortcomings in the Current Amyotrophic Lateral Sclerosis Trials and Potential Solutions for Improvement. Front Neurol. 2017;8 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5626834/ [cited 17 Sep 2018]. - PMC - PubMed
-
- Paul Mehta M. Prevalence of Amyotrophic Lateral Sclerosis — United States, 2012–2013. MMWR Surveill Summ. 2016;65 Available from: https://www.cdc.gov/mmwr/volumes/65/ss/ss6508a1.htm [cited 17 Sep 2018]. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

