Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants
- PMID: 30548483
- PMCID: PMC6516999
- DOI: 10.1002/14651858.CD012519.pub2
Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants
Abstract
Background: Inflammation may contribute to preterm birth and to morbidity of preterm infants. Preterm infants are at risk for alterations in the normal protective microbiome. Oral probiotics administered directly to preterm infants have been shown to decrease the risk for severe necrotizing enterocolitis (NEC) as well as the risk of death, but there are safety concerns about administration of probiotics directly to preterm infants. Through decreasing maternal inflammation, probiotics may play a role in preventing preterm birth and/or decreasing the inflammatory milieu surrounding delivery of preterm infants, and may alter the microbiome of the preterm infant when given to mothers during pregnancy. Probiotics given to mothers after birth of preterm infants may effect infant bacterial colonization, which could potentially reduce the incidence of NEC.
Objectives: 1. To compare the efficacy of maternal probiotic administration versus placebo or no intervention in mothers during pregnancy for the prevention of preterm birth and the prevention of morbidity and mortality of infants born preterm.2. To compare the efficacy of maternal probiotic administration versus placebo, no intervention, or neonatal probiotic administration in mothers of preterm infants after birth on the prevention of mortality and preterm infant morbidities such as NEC.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 2), MEDLINE via PubMed (1966 to 21 March 2017), Embase (1980 to 21 March 2017), and CINAHL (1982 to 21 March 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi-randomized trials.
Selection criteria: We included randomized controlled trials in the review if they administered oral probiotics to pregnant mothers at risk for preterm birth, or to mothers of preterm infants after birth. Quasi-randomized trials were eligible for inclusion, but none were identified. Studies enrolling pregnant women needed to administer probiotics at < 36 weeks' gestation until the trimester of birth. Probiotics considered were of the genera Lactobacillus, Bifidobacterium or Saccharomyces.
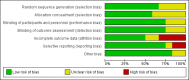
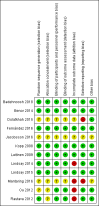
Data collection and analysis: We used the standard methods of the Cochrane Collaboration and Cochrane Neonatal to determine the methodologic quality of studies, and for data collection and analysis.
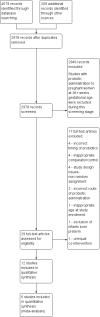
Main results: We included 12 eligible trials with a total of 1450 mothers and 1204 known infants. Eleven trials administered probiotics to mothers during pregnancy and one trial administered probiotics to mothers after birth of their preterm infants. No studies compared maternal probiotic administration directly with neonatal administration. Included prenatal trials were highly variable in the indication for the trial, the gestational age and duration of administration of probiotics, as well as the dose and formulation of the probiotics. The pregnant women included in these trials were overall at low risk for preterm birth. In a meta-analysis of trial data, oral probiotic administration to pregnant women did not reduce the incidence of preterm birth < 37 weeks (typical risk ratio (RR) 0.92, 95% confidence interval (CI) 0.32 to 2.67; 4 studies, 518 mothers and 506 infants), < 34 weeks (typical risk difference (RD) 0.00, 95% CI -0.02 to 0.02; 2 studies, 287 mothers and infants), the incidence of infant mortality (typical RD 0.00, 95% CI -0.02 to 0.02; 2 studies, 309 mothers and 298 infants), or the gestational age at birth (mean difference (MD) 0.15, 95% CI -0.33 to 0.63; 2 studies, 209 mothers with 207 infants).One trial studied administration of probiotics to mothers after preterm birth and included 49 mothers and 58 infants. There were no significant differences in the risk of any NEC (RR 0.44, 95% CI 0.13 to 1.46; 1 study, 58 infants), surgery for NEC (RR 0.15, 95% CI 0.01 to 2.58; 1 study, 58 infants), death (RR 0.66, 95% CI 0.06 to 6.88; 1 study, 58 infants), and death or NEC (RR 0.53, 95% CI 0.19 to 1.49; 1 study, 58 infants). There was an improvement in time to reach 50% enteral feeds in infants whose mothers received probiotics, but the estimate is imprecise (MD -9.60 days, 95% CI -19.04 to -0.16 days; 58 infants). No other improvement in any neonatal outcomes were reported. The estimates were imprecise and do not exclude the possibility of meaningful harms or benefits from maternal probiotic administration. There were no cases of culture-proven sepsis with the probiotic organism. The GRADE quality of evidence was judged to be low to very low due to inconsistency and imprecision.
Authors' conclusions: There is insufficient evidence to conclude whether there is appreciable benefit or harm to neonates of either oral supplementation of probiotics administered to pregnant women at low risk for preterm birth or oral supplementation of probiotics to mothers of preterm infants after birth. Oral supplementation of probiotics to mothers of preterm infants after birth may decrease time to 50% enteral feeds, however, this estimate is extremely imprecise. More research is needed for post-natal administration of probiotics to mothers of preterm infants, as well as to pregnant mothers at high risk for preterm birth.
Conflict of interest statement
JG has no interests to declare.
MB has no interests to declare.
RS has no interests to declare.
Figures
Update of
- doi: 10.1002/14651858.CD012519
Similar articles
-
Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2020 Mar 31;3(3):CD007137. doi: 10.1002/14651858.CD007137.pub6. Cochrane Database Syst Rev. 2020. PMID: 32232984 Free PMC article.
-
Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2017 Jun 28;6(6):CD007137. doi: 10.1002/14651858.CD007137.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Mar 31;3:CD007137. doi: 10.1002/14651858.CD007137.pub6. PMID: 28658720 Free PMC article. Updated.
-
Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants.Cochrane Database Syst Rev. 2020 Oct 15;10(10):CD005496. doi: 10.1002/14651858.CD005496.pub5. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Jul 26;7:CD005496. doi: 10.1002/14651858.CD005496.pub6. PMID: 33058137 Free PMC article. Updated.
-
Probiotics for prevention of necrotizing enterocolitis in preterm infants.Evid Based Child Health. 2014 Sep;9(3):584-671. doi: 10.1002/ebch.1976. Evid Based Child Health. 2014. PMID: 25236307 Review.
-
Inositol in preterm infants at risk for or having respiratory distress syndrome.Cochrane Database Syst Rev. 2019 Jul 8;7(7):CD000366. doi: 10.1002/14651858.CD000366.pub4. Cochrane Database Syst Rev. 2019. PMID: 31283839 Free PMC article.
Cited by
-
Preterm neonatal immunology at the intestinal interface.Cell Mol Life Sci. 2020 Apr;77(7):1209-1227. doi: 10.1007/s00018-019-03316-w. Epub 2019 Oct 1. Cell Mol Life Sci. 2020. PMID: 31576423 Free PMC article. Review.
-
Maternal and infant probiotic administration for morbidity of very low birth weight infants: a three-arm randomized placebo-controlled trial.Eur J Nutr. 2022 Oct;61(7):3637-3648. doi: 10.1007/s00394-022-02905-z. Epub 2022 May 31. Eur J Nutr. 2022. PMID: 35639151 Clinical Trial.
-
The reproductive tract microbiota in pregnancy.Biosci Rep. 2021 Sep 30;41(9):BSR20203908. doi: 10.1042/BSR20203908. Biosci Rep. 2021. PMID: 34397086 Free PMC article. Review.
-
Effect of probiotic administration to breastfeeding mothers with very low birthweight neonates on some neonatal and maternal outcomes: study protocol for a randomised, double-blind, placebo-controlled trial.BMJ Open. 2024 Aug 29;14(8):e079526. doi: 10.1136/bmjopen-2023-079526. BMJ Open. 2024. PMID: 39209790 Free PMC article.
-
Neonate Bloodstream Infections in Organization for Economic Cooperation and Development Countries: An Update on Epidemiology and Prevention.J Clin Med. 2019 Oct 21;8(10):1750. doi: 10.3390/jcm8101750. J Clin Med. 2019. PMID: 31640253 Free PMC article. Review.
References
References to studies included in this review
Badehnoosh 2018 {published data only}
-
- Badehnoosh B, Karamali M, Zarrati M, Jamilian M, Bahmani F, Tajabadi‐Ebrahimi M, et al. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. Journal of Maternal‐fetal & Neonatal Medicine 2018;31(9):1128‐36. [DOI: 10.1080/14767058.2017.1310193; PUBMED: 28326881] - DOI - PubMed
Benor 2014 {published data only}
-
- Benor S, Marom R, Ben Tov A, Armoni Domany K, Zaidenberg‐Israeli G, Dollberg S. Probiotic supplementation in mothers of very low birth weight infants. American Journal of Perinatology 2014;31(6):497‐504; Erratum in: American Journal of Perinatology: 2014: 31(6):e1. [DOI: 10.1055/s-0033-1353490; NCT00835874; PUBMED: 23934538] - DOI - PubMed
-
- Benor S, Marom R, Ben Tov A, Yarkoni I, Domany KA, Zaidenberg‐Israeli GA, et al. Probiotic supplementation in mothers of preterm infants: a randomized double blind, placebo controlled trial. Pediatric Academic Societies Annual Meeting; 2011 April 30‐ May 03; Denver, Colorado USA. 2011.
-
- NCT00835874. Probiotic administration to mothers of preterm infants to prevent necrotizing enterocolitis and sepsis. clinicaltrials.gov/show/NCT00835874 (first received 04 February 2009). [NCT00835874]
Dolatkhah 2015 {published data only}
-
- Dolatkhah N, Hajifaraji M, Abbasalizadeh F, Aghamohammadzadeh N, Mehrabi Y, Mesgari Abbasi M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. Journal of Health, Population, and Nutrition 2015;33(25):1‐8. [DOI: 10.1186/s41043-015-0034-9; PUBMED: 26825666] - DOI - PMC - PubMed
Fernández 2016 {published data only}
-
- Fernández L, Cárdenas N, Arroyo R, Manzano S, Jiménez E, Martín V, et al. Prevention of infectious mastitis by oral administration of lactobacillus salivarius PS2 during late pregnancy. Clinical Infectious Diseases 2016;62(5):568‐73. [DOI: 10.1093/cid/civ974; NCT01505361; PUBMED: 26611780] - DOI - PubMed
Jacobsson 2016 {published data only}
-
- Jacobsson B, Sengpiel V, Nilsson S, Hallingstrom M, Elfvin A, Brantsaeter AL, et al. Immunological and inflammatory response after antenatal supplementation with lactobacillus rhamnosus in low‐risk pregnant women. Reproductive Sciences 2016;23(Suppl 1):293A.
Kopp 2008 {published data only}
-
- Kopp MV, Goldstein M, Dietschek A, Sofke J, Heinzmann A, Urbanek R. Lactobacillus GG has in vitro effects on enhanced interleukin‐10 and interferon‐gamma release of mononuclear cells but no in vivo effects in supplemented mothers and their neonates. Clinical and Experimental Allergy 2008;38(4):602‐10. [DOI: 10.1111/j.1365-2222.2007.02911.x; PUBMED: 18167121] - DOI - PubMed
Laitinen 2009 {published data only}
-
- Aaltonen J, Ojala T, Laitinen K, Piirainen TJ, Poussa TA, Isolauri E. Evidence of infant blood pressure programming by maternal nutrition during pregnancy: a prospective randomized controlled intervention study. Journal of Pediatrics 2008;152(1):79‐84. [DOI: 10.1016/j.jpeds.2007.05.048; PUBMED: 18154905] - DOI - PubMed
-
- Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming:a prospective randomized controlled study. European Journal of Clinical Nutrition 2011;65(1):10‐9. [DOI: 10.1038/ejcn.2010.225; PUBMED: 20948557] - DOI - PubMed
-
- Hoppu U, Isolauri E, Laakso P, Matomäki J, Laitinen K. Probiotics and dietary counselling targeting maternal dietary fat intake modifies breast milk fatty acids and cytokines. European Journal of Nutrition 2012;51(2):211‐9. [DOI 10.1007/s00394‐011‐0209‐0; PUBMED: 21626296] - PubMed
-
- Huurre A, Laitinen K, Rautava S, Korkeamäki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double‐blind placebo‐controlled study. Clinical and Experimental Allergy 2008;38(8):1342‐8. [DOI: 10.1111/j.1365-2222.2008.03008.x; PUBMED: 18477013] - DOI - PubMed
Lindsay 2014 {published data only}
-
- ISRCTN97241163. Probiotics in pregnancy study (ProP Study). isrctn.com/ISRCTN97241163 (first received 15 February 2012). [DOI: 10.1186/ISRCTN97241163] - DOI
-
- Lindsay K, Brennan L, Kennelly M, Curran S, Coffey M, Smith T, et al. Influence of maternal metabolic response to gestational diabetes treatment on neonatal outcomes. American Journal of Obstetrics and Gynecology 2015;212(1):S247‐8. - PubMed
-
- Lindsay K, Maguire O, Smith T, Brennan L, McAuliffe F. A randomized controlled trial of probiotics to reduce maternal glycaemia in obese pregnancy. American Journal of Obstetrics & Gynecology 2014;210(1):S342.
-
- Lindsay KL, Brennan L, McAuliffe FM. Acceptability of and compliance with a probiotic capsule intervention in pregnancy. International Journal of Gynecology and Obstetrics 2014;125:279‐84. - PubMed
-
- Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose:a double‐blind, placebo‐controlled, randomized trial (Probiotics in Pregnancy Study). American Journal of Clinical Nutrition 2014;99(6):1432‐9. [DOI: 10.3945/ajcn.113.079723; PUBMED: 24646819] - DOI - PubMed
Lindsay 2015 {published data only}
-
- Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, Curran S, et al. Impact of probiotics in women with gestationaldiabetes mellitus on metabolic health: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(496):e1‐11. [DOI: 10.1016/j.ajog.2015.02.008; PUBMED: 25687568] - DOI - PubMed
Mantaring 2016 {published data only}
-
- Guinto VT, Destura R, Volger S, Vidal K, Pecquet S, Mantaring III JV. Randomized controlled study of nutritional supplement beverages with and without probiotics taken during the third trimester of pregnancy: effects on maternal and fetal outcomes and fetal immune status. International Journal of Gynaecology and Obstetrics 2015;131(Suppl 5):E314.
-
- Mantaring J, Benyacoub J, Destura R, Pecquet S, Vidal K, Volger S, et al. A nutritional supplement beverage with and without probiotics during the third trimester of pregnancy and lactation show benefit on infant growth up to 12 months of age in the Philippines. Journal of Pediatric Gastroenterology and Nutrition 2016;62(Suppl 1):706.
Ou 2012 {published data only}
-
- Kuo H, Yang K, Ou C. Antenatal probiotics reduces maternal but not childhood atopic diseases: a randomised, double‐blind, placebo‐controlled trial. Allergy 2012;67(Suppl 96):66‐7. - PubMed
-
- Ou C, Kuo HC, Wang L, Hsu TY, Chuang H, Liu CA, et al. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double‐blind, placebo‐controlled trial. Clinical and Experimental Allergy 2012;42(9):1386‐96. [DOI: 10.1111/j.1365-2222.2012.04037.x; NCT00325273; PUBMED: 22925325] - DOI - PubMed
Rautava 2012 {published data only}
References to studies excluded from this review
Asemi 2011 {published data only}
-
- Asemi A, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: a randomized controlled trial. Pakistan Journal of Biological Sciences: PJBS 2011;14(8):476‐82. [PUBMED: 21936251] - PubMed
Asemi 2012 {published data only}
-
- Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. Journal of Maternal‐fetal & Neonatal Medicine 2012;25(9):1552‐6. [DOI: 10.3109/14767058.2011.640372; PUBMED: 22098090] - DOI - PubMed
Bisanz 2014 {published data only}
-
- Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, et al. Randomized open‐label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 2014;5(5):e01580‐14. [DOI: 10.1128/mBio.01580-14; PUBMED: 25293764 ] - DOI - PMC - PubMed
Daskalakis 2014 {published data only}
-
- Daskalakis G, Karampelas A, Gavrili V, Papantoniou N, Angelopoulos P, Ntomali A, et al. Probiotics for preterm premature rupture of membranes. Journal of Maternal‐fetal & Neonatal Medicine 2014;27(Suppl 1):370. [DOI: 10.3109/14767058.2014.924236] - DOI
Gonai 2014 {published data only}
-
- Gonai M, Nakadaira I, Kurasaki K, Hamano K. The effects of lactobacilli on glycemic control and the secretion of glucagon‐like peptide‐1 in Japanese gestational diabetes mellitus patients. Diabetes 2014;63(Suppl 1):A3333.
Gronlund 2011 {published data only}
Grzeskowiak 2012 {published data only}
-
- Grzeskowiak L, Grönlund MM, Beckmann C, Salminen S, Berg A, Isolauri E. The impact of perinatal probiotic intervention on gut microbiota: double‐blind placebo‐controlled trials in Finland and Germany. Anaerobe 2012;18(1):7‐13. [DOI: 10.1016/j.anaerobe.2011.09.006; NCT00167700; PUBMED: 21979491] - DOI - PubMed
Hanson 2014 {published data only}
-
- Hanson L, VandeVusse L, Duster M, Warrack S, Safdar N. Feasibility of oral prenatal probiotics against maternal group B streptococcus vaginal and rectal colonization. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2014;43(3):294‐304. [DOI: 10.1111/1552-6909.12308; PUBMED: 24754328] - DOI - PubMed
-
- Hanson L, VandeVusse L, Safdar N, Duster M, Warrack S, Panjikar P. Effects of probiotic use during pregnancy on lactobacillus and Group B streptococcus vaginal colonization: pilot results. Journal of Midwifery & Women's Health 2012;57(5):537.
-
- Warrack SR, Hanson L, Vusse LV, Duster M, Panjikar P, Safdar N. The impact of prenatal probiotics on group B streptococcus colonization. American Journal of Infection Control 2013;41(6 Suppl 1):S142.
Hantoushzadeh 2012 {published data only}
-
- Hantoushzadeh S, Golshahi F, Javadian P, Khazardoost S, Aram S, Hashemi S, et al. Comparative efficacy of probiotic yoghurt and clindamycin in treatment of bacterial vaginosis in pregnant women: a randomized clinical trial. Journal of Maternal‐fetal & Neonatal Medicine 2012;25(7):1021‐4. [DOI: 10.3109/14767058.2011.614654; PUBMED: 21854132] - DOI - PubMed
Jain 2017 {published data only}
-
- Jain SK, Jain S, Saade G. Safety and efficacy of oral probiotics in pregnant women. American Journal of Obstetrics and Gynecology 2017;216(1 Supple 1):S523‐4.
Jamilian 2016 {published data only}
-
- Jamilian M, Bahmani F, Vahedpoor Z, Salmani A, Tajabadi‐Ebrahimi M, Jafari P. Effects of probiotic supplementation on metabolic status in pregnant women: a randomized, double‐blind, placebo‐controlled trial. Archives of Iranian Medicine 2016;19(10):687‐92. [DOI: ; PUBMED: 27743432] - PubMed
Karampelas 2013 {published data only}
-
- Karampelas A, Daskalakis G, Papantoniou N, Gavrili V, Mesogitis S, Angelopoulos P, et al. Probiotics for preterm premature rupture of membranes. Journal of Perinatal Medicine 2013;41(Suppl 1):205.
Krauss‐Silva 2011 {published data only}
-
- Krauss‐Silva L, Moreira ME, Alves MB, Braga A, Camacho KG, Batista MR. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results. Trials 2011;12:239. [DOI: 10.1186/1745-6215-12-239; PUBMED: 22059409] - DOI - PMC - PubMed
-
- Krauss‐Silva L, Moreira MEL, Alves MB, Rezende MR, Braga A, Camacho KG, et al. Randomized controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with intrauterine infection: study protocol. Reproductive Health 2010;7:14. [DOI: 10.1186/1742-4755-7-14; PUBMED: 20591191] - DOI - PMC - PubMed
-
- NCT00303082. Probiotics for the prevention of premature birth and neonatal related morbidity. clinicaltrials.gov/show/NCT00303082 (first received 15 March 2006). [NCT00303082]
Nishijima 2005 {published data only}
-
- Nishijima K, Shukunami K, Kotsuji F. Probiotics affects vaginal flora in pregnant women, suggesting the possibility of preventing preterm labor. Journal of Clinical Gastroenterology 2005;39(5):447‐8. [PUBMED: 15815217] - PubMed
Ortiz‐Andrellucchi 2008 {published data only}
-
- Ortiz‐Andrellucchi A, Sánchez‐Villegas A, Rodríguez‐Gallego C, Lemes A, Molero T, Soria A, et al. Immunomodulatory effects of the intake of fermented milk with Lactobacillus casei DN114001 in lactating mothers and their children. British Journal of Nutrition 2008;100(4):834‐45. [DOI: 10.1017/S0007114508959183; PUBMED: 18341756] - DOI - PubMed
Vitali 2012 {published data only}
-
- Vitali B, Cruciani F, Baldassarre ME, Capursi T, Spisni E, Valerii MC. Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiology 2012;12:12. [DOI: 10.1186/1471-2180-12-236; NCT01367470; PUBMED: 23078375] - DOI - PMC - PubMed
Wickens 2008 {published data only}
-
- Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. Probiotic Study Group. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double‐blind, randomized, placebo controlled trial. Journal of Allergy and Clinical Immunology 2008;122(4):788‐94. [DOI: 10.1016/j.jaci.2008.07.011; PUBMED: 18762327] - DOI - PubMed
-
- Wickens K, Stanley TV, Mitchell EA, Barthow C, Fitzharris P, Purdie G. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization?. Clinical and Experimental Allergy 2013;43(9):1048‐57. [DOI: 10.1111/cea.12154; PUBMED: 23957340] - DOI - PubMed
References to ongoing studies
ACTRN12611001208998 {published data only}
-
- ACTRN12611001208998. Probiotics for the prevention of gestational diabetes in overweight and obese women [Randomized placebo controlled trial of probiotics in overweight and obese women to assess the prevention of gestational diabetes]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=347738&isReview... (first received 21 November 2011).
-
- Nitert MD, Barrett HL, Foxcroft K, Tremellen A, Wilkinson S, Lingwood B, et al. SPRING: an RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy and Childbirth 2013;13(50):1‐7. [DOI: 10.1186/1471-2393-13-50; PUBMED: 23442391] - DOI - PMC - PubMed
ACTRN12612000196842 {published data only}
-
- ACTRN12612000196842. A randomized placebo controlled trial of the effects of the probiotic Lactobacillus rhamnosus HN001taken from the 1st trimester of pregnancy till 6 months post partum, if breastfeeding, on the development of eczema and atopic sensitization in infants by age 12 months. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362049&isReview... (first received 07 Feburary 2012).
-
- Barthow C, Wickens K, Stanley T, Mitchell EA, Robyn Maude R, Abels P, et al. The Probiotics in Pregnancy Study (PiPStudy): rationale and design of a double blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy and Childbirth 2016; Vol. 16, issue 1:133. [DOI: 10.1186/s12884-016-0923-y; PUBMED: 27255079] - DOI - PMC - PubMed
ACTRN12615000400561 {published data only}
-
- ACTRN12615000400561. A randomised controlled trial of nutritional interventions in obese pregnant women to optimise maternal pregnancy weight gain and infant birthweight: a demonstration study. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368302&isReview... (first received 10 April 2015).
-
- Okesene‐Gafa K, LI M, Taylor RS, Thompson JM, Crowther CA, McKinlay CJ, et al. A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial. BMC Pregnancy and Childbirth 2016;16(1):373. [DOI: 10.1186/s12884-016-1149-8; PUBMED: 27884128] - DOI - PMC - PubMed
CTRI/2013/04/003577 {published data only}
-
- CTRI/2013/04/003577. Effects of oral probiotic supplementation during pregnancy and lactation in modulating immune response in breast milk, fetus, and neonate‐ a randomised double blind controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/04/003577 (first received 23 April 2013).
ISRCTN53023014 {published data only}
-
- ISRCTN53023014. To study the transit of beneficial bacteria from mother to infant. isrctn.com/ISRCTN53023014 (first received 16 August 2016).
Mehta 2016 {published data only}
-
- Mehta S, DuPlessis J, Sunanda G, Darragh H. Randomized double‐blind placebo‐controlled trial of probiotics in pregnancy (PIP) and its effect on Group‐B streptococcal colonization‐ study protocol. Journal of Paediatrics and Child Health 2016;52(Supple 2):68.
NCT00217308 {published data only}
-
- NCT00217308. Effect of probiotic lactobacilli on vaginal flora of pregnant women at high risk for preterm delivery. clinicaltrials.gov/show/NCT00217308 (first received 22 September 2005).
NCT01436448 {published data only}
-
- NCT01436448. Probiotics (Lactobacillus Rhamnosus) in reducing glucose intolerance during and after pregnancy(GRIP). clinicaltrials.gov/show/NCT01436448 (first received 19 September 2011).
NCT01454661 {published data only}
-
- NCT01454661. Probiotics and early microbial contact in preterm neonates (ProPre). clinicaltrials.gov/show/NCT01454661 (first received 19 October 2011).
NCT01479478 {published data only}
-
- NCT01479478. Effects of oral probiotic supplementation on group B strep (GBS) rectovaginal colonization in pregnancy. clinicaltrials.gov/show/NCT01479478 (first received 24 November 2011).
NCT01577108 {published data only}
-
- NCT01577108. Oral probiotics reduce group B streptococci colonization in pregnant women. clinicaltrials.gov/show/NCT01577108 (first received 13 April 2012).
NCT01697683 {published data only}
-
- NCT01697683. Probiotic therapy for the reversal of bacterial vaginosis in pregnancy. clinicaltrials.gov/show/NCT01697683 (first received 02 October 2014).
NCT01779193 {published data only}
-
- NCT01779193. Probiotics capsule as supplemental therapy for group B Streptococci infection and vaginitis during pregnancy. Clinicaltrials.gov/show/ NCT01779193 (first received 30 January 2013).
NCT01922791 {published data only}
-
- NCT01922791. Nutrition and pregnancy intervention study. Clinicaltrials.gov/show/NCT01922791 (first received 14 August 2013).
NCT02377544 {published data only}
-
- NCT02377544. The role of Bifidobacterium Animalis ssp Lactis DR10 supplementation in women during pregnancy and lactation on breast milk IL‐8 and gut mucosa integrity in infant. Clinicaltrials.gov/show/NCT02377544 (first received 03 March 2015).
NCT02430246 {published data only}
-
- NCT02430246. The association between the transfer of lactobacilli from the gastrointestinal tract to the vagina and the prevention/ eradication of abnormal vaginal flora in high risk pregnancies. Clinicaltrials.gov/show/NCT02430246 (first received 30 April 2015).
NCT02508844 {published data only}
-
- NCT02508844. Effect of probiotics (Vivomixx®) on weight, microbiota and glucose tolerance in obese pregnant women and their newborn (POP). Clinicaltrials.gov/show/NCT02508844 (first received 27 July 2015).
NCT02509988 {published data only}
-
- NCT02509988. Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health (NiPPeR). clinicaltrials.gov/show/NCT02509988 (first received 10 August 2005).
NCT02528981 {published data only}
-
- NCT02528981. Effect of probiotics on GBS colonization status during pregnancy: a pilot randomized controlled trial. Clinicaltrials.gov/show/NCT02528981 (first received 19 August 2015).
NCT02637986 {published data only}
-
- NCT02637986. The efficacy of orally administrated probiotic formula in preventing a recurrence of a urinary tract infection during pregnancy. Clinicaltrials.gov/show/NCT02637986 (first received 22 December 2015).
NCT02692820 {unpublished data only}
-
- NCT02692820. Preventing preterm birth with probiotics (PrePro). Clinicaltrials.gov/show/NCT02692820 (first received 26 February 2016).
NCT02693028 {published data only}
-
- NCT02693028. Lactobacillus Reuteri feasibility study on probiotic treatment and perinatal microbiome. Clinicaltrials.gov/show/NCT02693028 (first received 26 February 2016).
NCT02693041 {published data only}
-
- NCT02693041. Pregnancy complications ‐ a probiotic interventional study. Clinicaltrials.gov/show/NCT02693041 (first received 26 February 2016).
NCT02768818 {published data only}
-
- NCT02768818. Modulation of the intestinal flora with the probiotic VIVOMIXX™ in pregnant women at risk of metabolic complications. Clinicaltrials.gov/show/NCT02768818 (first received 11 May 2016).
NCT02795845 {published data only}
-
- NCT02795845. Oral probiotics for the treatment and prevention of vulvovaginal infections in pregnancy ‐ double‐blind, randomized, placebo‐controlled study. Clinicaltrials.gov/show/NCT02795845 (first received 10 June 2016).
NCT02912416 {published data only}
-
- NCT02912416. Efficacy of probiotics on iron status during pregnancy. Clinicaltrials.gov/show/NCT02912416 (first received 23 September 2016).
NCT03008421 {published data only}
-
- NCT03008421. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Clinicaltrials.gov/show/NCT03008421 (first received 02 January 2017).
NCT03215784 {published data only}
-
- NCT03215784. Gestational obesity and interventions with probiotics or fish oil trial. Clinicaltrials.gov/show/NCT03215784 (first received 12 July 2017).
Additional references
Abrahamsson 2009
-
- Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Bjorksten B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. Journal of Pediatric Gastroenterology and Nutrition 2009;49(3):349–54. [DOI: 10.1097/MPG.0b013e31818f091b; PUBMED: 19525871] - DOI - PubMed
Agostoni 2010
-
- Agostoni C, Buonocore G, Carnielli VP, Curtis M, Darmaun D, Decsi T, et al. ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of PaediatricGastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85‐91. [DOI: 10.1097/MPG.0b013e3181adaee0; PUBMED: 19881390] - DOI - PubMed
AlFaleh 2014
Bell 1978
Bergmann 2014
Bertelli 2015
CDC 2015
-
- Vallabhaneni S, Walker TA, Lockhart SR, Ng D, Chiller T, Melchreit R, et al. Centers for Disease Control and Prevention (CDC). Notes from the Field: Fatal Gastrointestinal Mucormycosis in a Premature Infant Associated with a Contaminated Dietary Supplement — Connecticut, 2014. Centers for Disease Control and Prevention; Morbidity and Mortality Weekly Report (MMWR) February 20, 2015:https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6406a6.htm Accessed 6/15/18. - PMC - PubMed
CDC HAN 2014
-
- Center for Disease Control and Prevention Health Alert Network. Fatal gastrointestinal mucormycosis in an infant following ingestion of contaminated dietary supplement – Connecticut, 2014. emergency.cdc.gov/han/ (accessed 27 March 2018); Vol. CDCHAN‐00373.
Cilieborg 2012
Costeloe 2016
-
- Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MW, Probiotics in Preterm Infants Study Collaborative Group. Bifidobacterium breve BBG‐001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016;387(10019):649‐60. [DOI: 10.1016/S0140-6736(15)01027-2; PUBMED: 26628328] - DOI - PubMed
Deshpande 2010
Dugoua 2009
-
- Dugoua JJ, Machado M, Zhu X, Chen X, Koren G, Einarson TR. Probiotic safety in pregnancy: a systematic review and meta‐analysis of randomized controlled trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. Journal of Obstetrics and Gynaecology Canada 2009;31(6):542‐52. [DOI: 10.1016/S1701-2163(16)34218-9; PUBMED: 19646321] - DOI - PubMed
Elias 2011
Faa 2013
-
- Faa G, Gerosa C, Fanni D, Nemolato S, Eyken P, Fanos V. Factors influencing the development of a personal tailored microbiota in the neonate, with particular emphasis on antibiotic therapy. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(Suppl 2):35‐43. [DOI: 10.3109/14767058.2013.829700; PUBMED: 24059551] - DOI - PubMed
FDA 2014
-
- U.S. Food, Drug Administration. Dietary Supplements Containing Live Bacteria or Yeast in Immunocompromised Persons: Warning ‐ Risk of Invasive Fungal Disease. www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedica... 12/9/2014; Accessed in 2016.
Garland 2010
-
- Garland SM, Jacobs SE, Tobin JM, Opie GF, Donath S, ProPrems study group. A cautionary note on instituting probiotics into routine clinical care for premature infants. Pediatrics 2010;126(3):e741‐2; Erratum in: Pediatrics: 2010;126(5):1052. [DOI: 10.1542/peds.2010-1949B; PUBMED: 20810714] - DOI - PubMed
Gomez Arango 2015
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 27 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gueimonde 2006
-
- Gueimonde M, Sakata S, Kalliomaki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. Journal of Pediatric Gastroenterology and Nutrition 2006;42(2):166–70. [DOI: 10.1097/01.mpg.0000189346.25172.fd; PUBMED: 16456409] - DOI - PubMed
Hickey 2012
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2016
-
- Higgins RD, Saade G, Polin RA, Grobman WA, Buhimschi IR, Watterberg K, et al. Chorioamnionitis Workshop Participants. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstetrics & Gynecology 2016;127(3):426‐36. [DOI: 10.1097/AOG.0000000000001246; PUBMED: 26855098] - DOI - PMC - PubMed
ICCROP 2005
Jacobs 2013
Janvier 2013
Johnson 2012
Jost 2014
Keski‐Nisula 2013
Lahtinen 2009
-
- Lahtinen SJ, Boyle RJ, Kivivuori S, Oppedisano F, Smith KR, Robins‐Browne R, et al. Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. Journal of Allergy and Clinical Immunology 2009;123(2):499‐501.e8. [DOI: 10.1016/j.jaci.2008.11.034; PUBMED: 19135234] - DOI - PubMed
Lindsay 2013
-
- Lindsay KL, Walsh CA, Brennan L, McAuliffe FM. Probiotics in pregnancy and maternal outcomes: a systematic review. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(8):772‐8. [PUBMED: 10.3109/14767058.2012.755166; PUBMED: 23205866] - PubMed
Mackeen 2015
Matamaros 2013
Mihatsch 2012
-
- Mihatscha WA, Braeggerb CP, Decsic T, Kolacekd S, Lanzingere H, Mayerf B, et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clinical Nutrition 2012;31(1):6‐15. [DOI: 10.1016/j.clnu.2011.09.004; PUBMED: 21996513] - DOI - PubMed
Neu 2011
Othman 2007
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] - PubMed
Reid 2003
-
- Reid G, Bocking A. The potential for probiotics to prevent bacterial vaginosis and preterm labor. American Journal of Obstetrics and Gynecology 2003;189(4):1202‐8. [PUBMED: 14586379] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Soll 2010
The World Bank 2016
-
- The World Bank. World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed 27 March 2018).
Thomas 2010
VandeVusse 2013
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous