Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus
- PMID: 30549014
- PMCID: PMC6557583
- DOI: 10.1002/cphy.c170040
Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus
Abstract
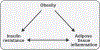
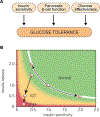
The objective of this comprehensive review is to summarize and discuss the available evidence of how adipose tissue inflammation affects insulin sensitivity and glucose tolerance. Low-grade, chronic adipose tissue inflammation is characterized by infiltration of macrophages and other immune cell populations into adipose tissue, and a shift toward more proinflammatory subtypes of leukocytes. The infiltration of proinflammatory cells in adipose tissue is associated with an increased production of key chemokines such as C-C motif chemokine ligand 2, proinflammatory cytokines including tumor necrosis factor α and interleukins 1β and 6 as well as reduced expression of the key insulin-sensitizing adipokine, adiponectin. In both rodent models and humans, adipose tissue inflammation is consistently associated with excess fat mass and insulin resistance. In humans, associations with insulin resistance are stronger and more consistent for inflammation in visceral as opposed to subcutaneous fat. Further, genetic alterations in mouse models of obesity that reduce adipose tissue inflammation are-almost without exception-associated with improved insulin sensitivity. However, a dissociation between adipose tissue inflammation and insulin resistance can be observed in very few rodent models of obesity as well as in humans following bariatric surgery- or low-calorie-diet-induced weight loss, illustrating that the etiology of insulin resistance is multifactorial. Taken together, adipose tissue inflammation is a key factor in the development of insulin resistance and type 2 diabetes in obesity, along with other factors that likely include inflammation and fat accumulation in other metabolically active tissues. © 2019 American Physiological Society. Compr Physiol 9:1-58, 2019.
Copyright © 2019 American Physiological Society. All rights reserved.
Figures
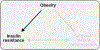




References
-
- Abate N, Sallam HS, Rizzo M, Nikolic D, Obradovic M, Bjelogrlic P and Isenovic ER. Resistin: an inflammatory cytokine. Role in cardiovascular diseases, diabetes and the metabolic syndrome. Curr Pharm Des 20: 4961–9, 2014. - PubMed
-
- Abdullah A, Peeters A, de Courten M and Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 89: 309–19, 2010. - PubMed
-
- Adamson SE, Griffiths R, Moravec R, Senthivinayagam S, Montgomery G, Chen W, Han J, Sharma PR, Mullins GR, Gorski SA, Cooper JA, Kadl A, Enfield K, Braciale TJ, Harris TE and Leitinger N. Disabled homolog 2 controls macrophage phenotypic polarization and adipose tissue inflammation. J Clin Invest 126: 1311–22, 2016. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

