An N-terminal heterozygous missense CASK mutation is associated with microcephaly and bilateral retinal dystrophy plus optic nerve atrophy
- PMID: 30549415
- PMCID: PMC6347510
- DOI: 10.1002/ajmg.a.60687
An N-terminal heterozygous missense CASK mutation is associated with microcephaly and bilateral retinal dystrophy plus optic nerve atrophy
Abstract
Heterozygous loss-of-function mutations in the X-linked gene CASK are associated with mental retardation and microcephaly with pontine and cerebellar hypoplasia (MICPCH) and ophthalmological disorders including optic nerve atrophy (ONA) and optic nerve hypoplasia (ONH). Recently, we have demonstrated that CASK(+/-) mice display ONH with 100% penetrance but exhibit no change in retinal lamination or structure. It is not clear if CASK loss-of-function predominantly affects retinal ganglion cells, or if other retinal cells like photoreceptors are also involved. Here, we report a heterozygous missense mutation in the N-terminal calcium/calmodulin-dependent kinase (CaMK) domain of the CASK protein in which a highly conserved leucine is mutated to the cyclic amino acid proline. In silico analysis suggests that the mutation may produce destabilizing structural changes. Experimentally, we observe pronounced misfolding and insolubility of the CASKL209P protein. Interestingly, the remaining soluble mutant protein fails to interact with Mint1, which specifically binds to CASK's CaMK domain, suggesting a mechanism for the phenotypes observed with the CASKL209P mutation. In addition to microcephaly, cerebellar hypoplasia and delayed development, the subject with the L209P mutation also presented with bilateral retinal dystrophy and ONA. Electroretinography indicated that rod photoreceptors are the most prominently affected cells. Our data suggest that the CASK interactions mediated by the CaMK domain may play a crucial role in retinal function, and thus, in addition to ONH, individuals with mutations in the CASK gene may exhibit other retinal disorders, depending on the nature of mutation.
Keywords: CASK; MICPCH; optic nerve atrophy; optic nerve hypoplasia; retinal dystrophy.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest
The authors have no conflicts of interest to report.
Figures


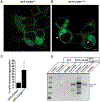
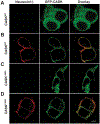

References
-
- Burglen L, Chantot-Bastaraud S, Garel C, Milh M, Touraine R, Zanni G, … Rodriguez D (2012). Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: confirmation of a recognizable phenotype and first description of a male mosaic patient. Orphanet J Rare Dis, 7, 18. doi:1750–1172-7–18 [pii]10.1186/1750-1172-7-18 - DOI - PMC - PubMed
-
- Butz S, Okamoto M, & Sudhof TC (1998). A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell, 94(6), 773–782. - PubMed
-
- Cibis GW, & Fitzgerald KM (1994). Optic nerve hypoplasia in association with brain anomalies and an abnormal electroretinogram. Doc Ophthalmol, 86(1), 11–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

