Early immune biomarkers and intermediate-term outcomes after heart transplantation: Results of Clinical Trials in Organ Transplantation-18
- PMID: 30549425
- PMCID: PMC6482086
- DOI: 10.1111/ajt.15218
Early immune biomarkers and intermediate-term outcomes after heart transplantation: Results of Clinical Trials in Organ Transplantation-18
Abstract
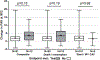
Clinical Trials in Organ Transplantation-18 (CTOT-18) is a follow-up analysis of the 200-subject multicenter heart transplant CTOT-05 cohort. CTOT-18 aimed to identify clinical, epidemiologic, and biologic markers associated with adverse clinical events past 1 year posttransplantation. We examined various candidate biomarkers including serum antibodies, angiogenic proteins, blood gene expression profiles, and T cell alloreactivity. The composite endpoint (CE) included death, retransplantation, coronary stent, myocardial infarction, and cardiac allograft vasculopathy. The mean follow-up was 4.5 ± SD 1.1 years. Subjects with serum anti-cardiac myosin (CM) antibody detected at transplantation and at 12 months had a higher risk of meeting the CE compared to those without anti-CM antibody (hazard ratio [HR] = 2.9, P = .046). Plasma VEGF-A and VEGF-C levels pretransplant were associated with CE (odds ratio [OR] = 13.24, P = .029; and OR = 0.13, P = .037, respectively). Early intravascular ultrasound findings or other candidate biomarkers were not associated with the study outcomes. In conclusion, anti-CM antibody and plasma levels of VEGF-A and VEGF-C were associated with an increased risk of adverse events. Although this multicenter report supports further evaluation of the mechanisms through which anti-CM antibody and plasma angiogenesis proteins lead to allograft injury, we could not identify additional markers of adverse events or potential novel therapeutic targets.
Keywords: alloantibody; clinical research/practice; heart (allograft) function/dysfunction; heart transplantation/cardiology; vasculopathy.
© 2018 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures


References
-
- Lund LH, Edwards LB, Dipchand AI, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016;35(10):1158–69. - PubMed
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. - PubMed
-
- Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr, Colombo PC, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med. 2017;376(5):440–50. - PubMed
-
- Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med. 2017;376(5):451–60. - PubMed
-
- Colvin M, Smith JM, Skeans MA, Edwards LB, Uccellini K, Snyder JJ, et al. OPTN/SRTR 2015 Annual Data Report: Heart. Am J Transplant. 2017;17 Suppl 1:286–356. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

