High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations
- PMID: 30550483
- PMCID: PMC6296248
- DOI: 10.1097/PGP.0000000000000491
High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations
Abstract
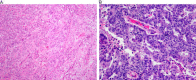
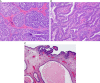
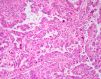
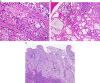
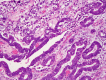
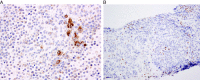
This review of challenging diagnostic issues concerning high-grade endometrial carcinomas is derived from the authors' review of the literature followed by discussions at the Endometrial Cancer Workshop sponsored by the International Society of Gynecological Pathologists in 2016. Recommendations presented are evidence-based, insofar as this is possible, given that the levels of evidence are weak or moderate due to small sample sizes and nonuniform diagnostic criteria used in many studies. High-grade endometrioid carcinomas include FIGO grade 3 endometrioid carcinomas, serous carcinomas, clear cell carcinomas, undifferentiated carcinomas, and carcinosarcomas. FIGO grade 3 endometrioid carcinoma is diagnosed when an endometrioid carcinoma exhibits >50% solid architecture (excluding squamous areas), or when an architecturally FIGO grade 2 endometrioid carcinoma exhibits marked cytologic atypia, provided that a glandular variant of serous carcinoma has been excluded. The most useful immunohistochemical studies to make the distinction between these 2 histotypes are p53, p16, DNA mismatch repair proteins, PTEN, and ARID1A. Endometrial clear cell carcinomas must display prototypical architectural and cytologic features for diagnosis. Immunohistochemical stains, including, Napsin A and p504s can be used as ancillary diagnostic tools; p53 expression is aberrant in a minority of clear cell carcinomas. Of note, clear cells are found in all types of high-grade endometrial carcinomas, leading to a tendency to overdiagnose clear cell carcinoma. Undifferentiated carcinoma (which when associated with a component of low-grade endometrioid carcinoma is termed "dedifferentiated carcinoma") is composed of sheets of monotonous, typically dyscohesive cells, which can have a rhabdoid appearance; they often exhibit limited expression of cytokeratins and epithelial membrane antigen, are usually negative for PAX8 and hormone receptors, lack membranous e-cadherin and commonly demonstrate loss of expression of DNA mismatch repair proteins and SWI-SNF chromatin remodeling proteins. Carcinosarcomas must show unequivocal morphologic evidence of malignant epithelial and mesenchymal differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Fadare O, Parkash V, Dupont WD, et al. The diagnosis of endometrial carcinomas with clear cells by gynecologic pathologists: an assessment of interobserver variability and associated morphologic features. Am J Surg Pathol 2012;36:1107–18. - PubMed
-
- Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 2013;37:874–81. - PubMed
-
- Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 2013;26:1594–604. - PubMed
-
- Han G, Soslow RA, Wethington S, et al. Endometrial carcinomas with clear cells: a study of a heterogeneous group of tumors including interobserver variability, mutation analysis, and immunohistochemistry with HNF-1beta. Int J Gynecol Pathol 2015;34:323–33. - PubMed
-
- Hoang LN, McConechy MK, Kobel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 2013;37:1421–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

