A high throughput neutralization test based on GFP expression by recombinant rabies virus
- PMID: 30550592
- PMCID: PMC6310286
- DOI: 10.1371/journal.pntd.0007011
A high throughput neutralization test based on GFP expression by recombinant rabies virus
Abstract
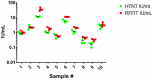
The effectiveness of rabies vaccination in both humans and animals is determined by the presence of virus neutralizing antibodies (VNAs). The Rapid Fluorescent Focus Inhibition Test (RFFIT) is the method traditionally used for detection and quantification of VNAs. It is a functional in vitro test for assessing the ability of antibodies in serum to bind and prevent infection of cultured cells with rabies virus (RABV). The RFFIT is a labor intensive, low throughput and semi-quantitative assay performed by trained laboratorians. It requires staining of RABV-infected cells by rabies specific fluorescent antibodies and manual quantification of fluorescent fields for titer determination. Although the quantification of fluorescent fields observed in each sample is recorded, the corresponding images are not stored or captured to be used for future analysis. To circumvent several of these disadvantages, we have developed an alternative, automated high throughput neutralization test (HTNT) for determination of rabies VNAs based on green fluorescent protein (GFP) expression by a recombinant RABV and compared with the RFFIT. The HTNT assay utilizes the recombinant RABV ERA variant expressing GFP with a nuclear localization signal (NLS) for efficient quantification. The HTNT is a quantitative method where the number of RABV-infected cells are determined and the images are stored for future analysis. Both RFFIT and HTNT results correlated 100% for a panel of human and animal positive and negative rabies serum samples. Although, the VNA titer values are generally agreeable, HTNT titers tend to be lower than that of RFFIT, probably due to the differences in quantification methods. Our data demonstrates the potential for HTNT assays in determination of rabies VNA titers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, et al. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57(RR-3):1–28. Epub 2008/05/23. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

