Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors
- PMID: 30550785
- PMCID: PMC6298040
- DOI: 10.1016/j.cell.2018.10.025
Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors
Erratum in
-
Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors.Cell. 2019 Jul 11;178(2):507-508. doi: 10.1016/j.cell.2019.06.028. Cell. 2019. PMID: 31299203 Free PMC article. No abstract available.
Abstract
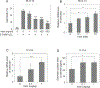
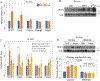
Irisin is secreted by muscle, increases with exercise, and mediates certain favorable effects of physical activity. In particular, irisin has been shown to have beneficial effects in adipose tissues, brain, and bone. However, the skeletal response to exercise is less clear, and the receptor for irisin has not been identified. Here we show that irisin binds to proteins of the αV class of integrins, and biophysical studies identify interacting surfaces between irisin and αV/β5 integrin. Chemical inhibition of the αV integrins blocks signaling and function by irisin in osteocytes and fat cells. Irisin increases both osteocytic survival and production of sclerostin, a local modulator of bone remodeling. Genetic ablation of FNDC5 (or irisin) completely blocks osteocytic osteolysis induced by ovariectomy, preventing bone loss and supporting an important role of irisin in skeletal remodeling. Identification of the irisin receptor should greatly facilitate our understanding of irisin's function in exercise and human health.
Keywords: Irisin receptor; bone resorption; integrin αV; irisin; osteocyte; sclerostin; subcutaneous (inguinal) adipose tissues; ucp1.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests
B.M.S., H.K., L.F.B., C.J.R., and R.B. have a pending patent on irisin.
Figures





Comment in
-
Irisin receptor in osteocytes identified.Nat Rev Endocrinol. 2019 Feb;15(2):63. doi: 10.1038/s41574-018-0151-9. Nat Rev Endocrinol. 2019. PMID: 30602738 No abstract available.
-
Boning Up on Irisin.N Engl J Med. 2019 Apr 11;380(15):1480-1482. doi: 10.1056/NEJMcibr1900041. N Engl J Med. 2019. PMID: 30970196 No abstract available.
References
-
- Baron R, and Kneissel M (2013). WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature medicine 19, 179–192. - PubMed
-
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, and Jilka RL (2005). Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146, 4577–4583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

