Lysophosphatidic acid receptor type 2 activation contributes to secondary damage after spinal cord injury in mice
- PMID: 30550929
- PMCID: PMC6348147
- DOI: 10.1016/j.bbi.2018.12.007
Lysophosphatidic acid receptor type 2 activation contributes to secondary damage after spinal cord injury in mice
Abstract
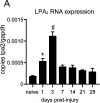
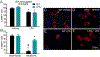
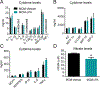
Lysophosphatidic acid (LPA) is an extracellular lipid mediator involved in many physiological functions by signaling through six known G-protein-coupled receptors (LPA1-LPA6). In the central nervous system (CNS), LPA mediates a wide range of effects, including neural progenitor cell physiology, astrocyte and microglia activation, neuronal cell death, axonal retraction, and contributions to pain, schizophrenia and hydrocephalus. We recently reported that LPA-LPA1 signaling mediates functional deficits and myelin loss after spinal cord injury (SCI). Here, we provide clear evidence on the deleterious contribution of another LPA receptor, LPA2, to myelin loss after SCI. We found that LPA2 is constitutively expressed in the spinal cord parenchyma and its transcripts were up-regulated after contusion injury, in part, by microglial cells. We also found that the demyelinating lesion triggered by intraspinal injection of LPA into the undamaged spinal cord was markedly reduced in the lack of LPA2. Similarly, LPA2 deficient mice showed enhanced motor skills and myelin sparing after SCI. To gain insights into the detrimental actions of LPA2 in spinal cord we performed cell culture studies. These experiments revealed that, similar to LPA1, activation of microglia LPA2 led to oligodendrocyte cell death. Moreover, we also found that the cytotoxic effects underlaying microglial LPA-LPA2 axis were mediated by the release of purines by microglia and the activation of P2X7 receptor on oligodendrocytes. Overall, this study provides new mechanistic insights into how LPA contributes to SCI physiopathology, and suggest that targeting LPA2 could be a novel therapeutic approach for the treatment of acute SCI.
Keywords: Demyelination; Inflammation; Lysophosphatidic acid; Microglia; Neurodegeneration; Spinal cord injury.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



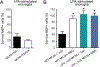
References
-
- Amo-Aparicio J, Martinez-Muriana A, Sanchez-Fernandez A, Lopez-Vales R, 2018. Neuroinflammation quantification for spinal cord injury. Curr. Protoc. Immunol 123:e57. - PubMed
-
- An S, Bleu T, Hallmark OG, Goetzl EJ, 1998. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem 273, 7906–7910. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG, 2006. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659. - PubMed
-
- Brosius Lutz A, Barres BA, 2014. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev. Cell 28, 7–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

