Cost-Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Pediatric Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia
- PMID: 30551196
- PMCID: PMC6624167
- DOI: 10.1093/jnci/djy193
Cost-Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Pediatric Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia
Abstract
Background: Chimeric antigen receptor T-cell (CAR-T) therapy is a promising new class of cancer therapy but has a high up-front cost. We evaluated the cost-effectiveness of CAR-T therapy among pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL).
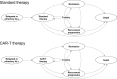
Methods: We built a microsimulation model for pediatric patients with relapsed/refractory B-ALL receiving either CAR-T therapy or standard of care. Outcomes included costs, quality of life (health utility), complications, and survival. We measured cost-effectiveness with the incremental cost-effectiveness ratio (ICER), with ICERs under $100 000 per quality-adjusted life-year (QALY) considered cost effective. One-way and probabilistic sensitivity analyses were used to test model uncertainty.
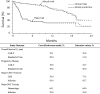
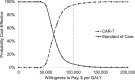
Results: Compared to standard of care, CAR-T therapy increased overall cost by $528 200 and improved effectiveness by 8.18 QALYs, resulting in an ICER of $64 600/QALY. The model was sensitive to assumptions about long-term CAR-T survival, the complete remission rate of CAR-T patients, and the health utility of long-term survivors. The base model assumed a 76.0% one-year survival with CAR-T, although if this decreased to 57.8%, then CAR-T was no longer cost effective. If the complete remission rate of CAR-T recipients decreased from 81% to 56.2%, or if the health utility of disease-free survivors decreased from 0.94 to 0.66, then CAR-T was no longer cost effective. Probabilistic sensitivity analysis found that CAR-T was cost effective in 94.8% of iterations at a willingness to pay of $100 000/QALY.
Conclusion: CAR-T therapy may represent a cost-effective option for pediatric relapsed/refractory B-ALL, although longer follow-up of CAR-T survivors is required to confirm validity of these findings.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Cost-Effective But Unaffordable: The CAR-T Conundrum.J Natl Cancer Inst. 2019 Jul 1;111(7):644-645. doi: 10.1093/jnci/djy195. J Natl Cancer Inst. 2019. PMID: 30561705 No abstract available.
References
-
- Escherich G, Horstmann MA, Zimmermann M, et al. Cooperative study group for childhood acute lymphoblastic leukaemia (COALL): long-term results of trials 82, 85, 89, 92 and 97. Leukemia. 2010;242:298–308. - PubMed
-
- Conter V, Aricò M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;242:255–264. - PubMed
-
- Stary J, Zimmermann M, Campbell M, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol. 2014;323:174–184. - PubMed

