The Effect of Prostaglandin Analogue Bimatoprost on Thyroid-Associated Orbitopathy
- PMID: 30551199
- PMCID: PMC6296211
- DOI: 10.1167/iovs.18-25134
The Effect of Prostaglandin Analogue Bimatoprost on Thyroid-Associated Orbitopathy
Abstract
Purpose: We characterize the effect of bimatoprost on orbital adipose tissue in thyroid-associated orbitopathy (TAO) with clinicopathologic correlation.
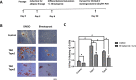
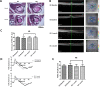
Methods: Orbital adipose-derived stem cells (OASCs) from types 1 and 2 TAO and control patients with and without exposure to 1 μm bimatoprost were examined via immunohistochemistry, RT-PCR, and Western blot for cell viability, migration capacity, lipid content, adipocyte morphology, mitochondrial content, and levels of adipogenic markers. A retrospective chart review was performed for clinicopathologic correlation. In mice, optical coherence tomography and pattern electroretinography were performed at baseline and at 1 month following a retrobulbar injection of bimatoprost, followed by orbital exenteration for histopathologic examination.
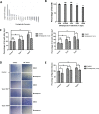
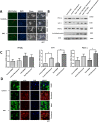
Results: Types 1 and 2 TAO-derived cells had a significantly higher migration capacity and lipid content than those of healthy controls. With the addition of bimatoprost, types 1 and 2 TAO and control adipocytes exhibited a significant decrease in lipid content with morphologic transformation into smaller and multilocular lipid droplets, and an increase in mitochondrial load and UCP-1 expression consistent with an increase in brown adipose tissue turnover. Retrobulbar injection of bimatoprost in mice did not alter the gross morphology, retinal thickness, or ganglion cell function in vivo.
Conclusions: Bimatoprost inhibits adipogenesis in OASCs and upregulates pathways involved in the browning of adipocytes. Furthermore, retrobulbar injection of bimatoprost is tolerated without immediate adverse effects in mice. Our results suggest a potential future application of prostaglandin analogues in the treatment of TAO.
Figures


Similar articles
-
In Vivo Effects of Retrobulbar Bimatoprost Injection on Orbital Fat.Ophthalmic Plast Reconstr Surg. 2018 May/Jun;34(3):201-204. doi: 10.1097/IOP.0000000000000907. Ophthalmic Plast Reconstr Surg. 2018. PMID: 28369019 Free PMC article.
-
Insulin-like growth factor 1 promotes the proliferation and adipogenesis of orbital adipose-derived stromal cells in thyroid-associated ophthalmopathy.Exp Eye Res. 2013 Feb;107:65-73. doi: 10.1016/j.exer.2012.11.014. Epub 2012 Dec 5. Exp Eye Res. 2013. PMID: 23219871
-
In Vivo Effects of Prostaglandin Analogues Application by Topical Drops or Retrobulbar Injections on the Orbital Fat of a Rat Model.Ocul Immunol Inflamm. 2023 Feb;31(2):298-303. doi: 10.1080/09273948.2022.2026977. Epub 2022 Jan 26. Ocul Immunol Inflamm. 2023. PMID: 35081015
-
RNA-Sequencing Gene Expression Profiling of Orbital Adipose-Derived Stem Cell Population Implicate HOX Genes and WNT Signaling Dysregulation in the Pathogenesis of Thyroid-Associated Orbitopathy.Invest Ophthalmol Vis Sci. 2017 Dec 1;58(14):6146-6158. doi: 10.1167/iovs.17-22237. Invest Ophthalmol Vis Sci. 2017. PMID: 29214313 Free PMC article.
-
Traditional Chinese medicine in thyroid-associated orbitopathy.J Endocrinol Invest. 2023 Jun;46(6):1103-1113. doi: 10.1007/s40618-023-02024-4. Epub 2023 Feb 12. J Endocrinol Invest. 2023. PMID: 36781592 Free PMC article. Review.
Cited by
-
Adipose Stem Cells in Modern-Day Ophthalmology.Clin Pract. 2023 Feb 4;13(1):230-245. doi: 10.3390/clinpract13010021. Clin Pract. 2023. PMID: 36826163 Free PMC article. Review.
-
The selection of target areas for orbital imaging, application of diethylenetriaminepentaacetic acid orbital imaging, clinical factors, alkaline phosphatase, and thyrotropin receptor antibodies in the staging and grading of thyroid-associated ophthalmopathy.Nucl Med Commun. 2025 Feb 1;46(2):152-161. doi: 10.1097/MNM.0000000000001934. Epub 2024 Nov 28. Nucl Med Commun. 2025. PMID: 39604281 Free PMC article.
-
Potential Therapeutic Activity of Berberine in Thyroid-Associated Ophthalmopathy: Inhibitory Effects on Tissue Remodeling in Orbital Fibroblasts.Invest Ophthalmol Vis Sci. 2022 Sep 1;63(10):6. doi: 10.1167/iovs.63.10.6. Invest Ophthalmol Vis Sci. 2022. PMID: 36094643 Free PMC article.
-
Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts.Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):13. doi: 10.1167/iovs.61.6.13. Invest Ophthalmol Vis Sci. 2020. PMID: 32503053 Free PMC article.
-
Thinking inside the box: Current insights into targeting orbital tissue remodeling and inflammation in thyroid eye disease.Surv Ophthalmol. 2022 May-Jun;67(3):858-874. doi: 10.1016/j.survophthal.2021.08.010. Epub 2021 Sep 4. Surv Ophthalmol. 2022. PMID: 34487739 Free PMC article. Review.
References
-
- Weetman AP. Graves' disease. N Engl J Med. 2000;343:1236–1248. - PubMed
-
- Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2000;85:1194–1199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

