A-to-I RNA Editing Affects lncRNAs Expression after Heat Shock
- PMID: 30551666
- PMCID: PMC6315331
- DOI: 10.3390/genes9120627
A-to-I RNA Editing Affects lncRNAs Expression after Heat Shock
Abstract
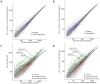
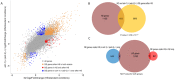
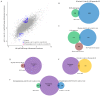
Adenosine to inosine (A-to-I) RNA editing is a highly conserved regulatory process carried out by adenosine-deaminases (ADARs) on double-stranded RNA (dsRNAs). Although a considerable fraction of the transcriptome is edited, the function of most editing sites is unknown. Previous studies indicate changes in A-to-I RNA editing frequencies following exposure to several stress types. However, the overall effect of stress on the expression of ADAR targets is not fully understood. Here, we performed high-throughput RNA sequencing of wild-type and ADAR mutant Caenorhabditis elegans worms after heat-shock to analyze the effect of heat-shock stress on the expression pattern of genes. We found that ADAR regulation following heat-shock does not directly involve heat-shock related genes. Our analysis also revealed that long non-coding RNAs (lncRNAs) and pseudogenes, which have a tendency for secondary RNA structures, are enriched among upregulated genes following heat-shock in ADAR mutant worms. The same group of genes is downregulated in ADAR mutant worms under permissive conditions, which is likely, considering that A-to-I editing protects endogenous dsRNA from RNA-interference (RNAi). Therefore, temperature increases may destabilize dsRNA structures and protect them from RNAi degradation, despite the lack of ADAR function. These findings shed new light on the dynamics of gene expression under heat-shock in relation to ADAR function.
Keywords: ADAR; Caenorhabditis elegans; stress response; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

