Implementing a comprehensive translational oncology platform: from molecular testing to actionability
- PMID: 30551737
- PMCID: PMC6295039
- DOI: 10.1186/s12967-018-1733-y
Implementing a comprehensive translational oncology platform: from molecular testing to actionability
Abstract
Background: In order to establish the workflows required to implement a real-time process involving multi-omic analysis of patient samples to support precision-guided therapeutic intervention, a tissue acquisition and analysis trial was implemented. This report describes our findings to date, including the frequency with which mutational testing led to precision-guided therapy and outcome for those patients.
Methods: Eligible patients presenting to Oregon Health and Science University Knight Cancer Institute were enrolled on the study. Patients with biopsy proven metastatic or locally advanced unresectable prostate cancer, breast cancer, pancreatic adenocarcinoma, or refractory acute myelogenous leukemia receiving standard of care therapy were eligible. Metastatic site biopsies were collected and analyzed using the Knight Diagnostic Lab GeneTrails comprehensive solid tumor panel (124 genes). CLIA certified genomic information was made available to the treating physician.
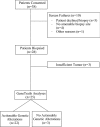
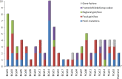
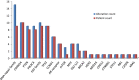
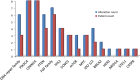
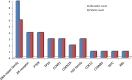
Results: Between 1/26/2017 and 5/30/2018, 38 patients were enrolled, with 28 successfully undergoing biopsy. Of these, 25 samples yielded sufficient tumor for analysis. The median biopsy cellularity and number of cores collected were 70% (15-90%) and 5 (2-20), respectively. No procedure-related complications occurred. GeneTrails analysis revealed that 22 of 25 (88%) tumor samples harbored at least one potentially actionable mutation, and 18 (72%) samples harbored 2 or more potentially actionable mutations. The most common genetic alterations identified involved: DNA damage repair genes, cell cycle regulating genes, PIK3CA/Akt/mTOR pathway, and FGF gene family. To date, CLIA certified genomic results were used by treating physicians for precision-guided therapy in 5 (23%) patients.
Conclusion: We report the feasibility of real-time tissue acquisition and analysis to support a successful translational oncology platform. The workflow will provide the foundation to improve access and accrual to biomarker driven precision oncology trials.
Keywords: Biomarkers; Clinical trials; Precision oncology; Targeted therapy; Translational research.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

