Clinical, immunological and bacteriological characteristics of H7N9 patients nosocomially co-infected by Acinetobacter Baumannii: a case control study
- PMID: 30551738
- PMCID: PMC6295110
- DOI: 10.1186/s12879-018-3447-4
Clinical, immunological and bacteriological characteristics of H7N9 patients nosocomially co-infected by Acinetobacter Baumannii: a case control study
Abstract
Background: Bacterial co-infection of patients suffering from influenza pneumonia is a key element that increases morbidity and mortality. The occurrence of Acinetobacter baumannii co-infection in patients with avian influenza A (H7N9) virus infection has been described as one of the most prevalent bacterial co-infections. However, the clinical and laboratory features of this entity of H7N9 and A. baumannii co-infection have not been systematically investigated.
Methods: We collected clinical and laboratory data from laboratory-confirmed H7N9 cases co-infected by A. baumannii. H7N9 patients without bacterial co-infection and patients with A. baumannii-related pneumonia in the same hospital during the same period were recruited as controls. The antibiotic resistance features and the corresponding genome determinants of A. baumannii and the immune responses of the patients were tested through the respiratory and peripheral blood specimens.
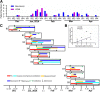
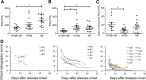
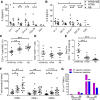
Results: Invasive mechanical ventilation was the most significant risk factor for the nosocomial A. baumannii co-infection in H7N9 patients. The co-infection resulted in severe clinical manifestation which was associated with the dysregulation of immune responses including deranged T-cell counts, antigen-specific T-cell responses and plasma cytokines. The emergence of genome variations of extensively drug-resistant A. baumannii associated with acquired polymyxin resistance contributed to the fatal outcome of a co-infected patient.
Conclusions: The co-infection of H7N9 patients by extensively drug-resistant A. baumannii with H7N9 infection is an important issue which deserves attention. The dysfunctions of immune responses were associated with the co-infection and were correlated with the disease severity. These data provide useful reference for the diagnosis and treatment of H7N9 infection.
Keywords: Acinetobacter baumannii; Avian influenza A(H7N9) virus; Extensively drug-resistant bacteria; Immune responses; Nosocomial infection; Pneumonia.
Conflict of interest statement
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
A case report demonstrating the utility of next generation sequencing in analyzing serial samples from the lung following an infection with influenza A (H7N9) virus.J Clin Virol. 2016 Mar;76:45-50. doi: 10.1016/j.jcv.2015.12.013. Epub 2016 Jan 4. J Clin Virol. 2016. PMID: 26826577
-
Serial high-resolution analysis of blood virome and host cytokines expression profile of a patient with fatal H7N9 infection by massively parallel RNA sequencing.Clin Microbiol Infect. 2015 Jul;21(7):713.e1-4. doi: 10.1016/j.cmi.2015.03.006. Epub 2015 Apr 13. Clin Microbiol Infect. 2015. PMID: 25882353
-
ARDS associated with pneumonia caused by avian influenza A H7N9 virus treated with extracorporeal membrane oxygenation.Clin Respir J. 2015 Jul;9(3):380-4. doi: 10.1111/crj.12140. Epub 2014 May 21. Clin Respir J. 2015. PMID: 24725670
-
Extracorporeal membrane oxygenation for avian influenza A (H7N9) patient with acute respiratory distress syndrome: a case report and short literature review.BMC Pulm Med. 2017 Feb 14;17(1):38. doi: 10.1186/s12890-017-0381-y. BMC Pulm Med. 2017. PMID: 28196469 Free PMC article. Review.
-
Drug treatment for multidrug-resistant Acinetobacter baumannii infections.Future Microbiol. 2008 Dec;3(6):649-60. doi: 10.2217/17460913.3.6.649. Future Microbiol. 2008. PMID: 19072182 Review.
Cited by
-
Avian influenza A (H7N9) virus: from low pathogenic to highly pathogenic.Front Med. 2021 Aug;15(4):507-527. doi: 10.1007/s11684-020-0814-5. Epub 2021 Apr 16. Front Med. 2021. PMID: 33860875 Free PMC article. Review.
-
The current landscape of microRNAs (miRNAs) in bacterial pneumonia: opportunities and challenges.Cell Mol Biol Lett. 2022 Aug 19;27(1):70. doi: 10.1186/s11658-022-00368-y. Cell Mol Biol Lett. 2022. PMID: 35986232 Free PMC article. Review.
-
Highly diverse sputum microbiota correlates with the disease severity in patients with community-acquired pneumonia: a longitudinal cohort study.Respir Res. 2024 May 29;25(1):223. doi: 10.1186/s12931-024-02821-2. Respir Res. 2024. PMID: 38811936 Free PMC article.
-
Acinetobacter baumannii: Its Clinical Significance in Human and Veterinary Medicine.Pathogens. 2021 Jan 27;10(2):127. doi: 10.3390/pathogens10020127. Pathogens. 2021. PMID: 33513701 Free PMC article. Review.
-
Unveiling Natural Power: Morin and Myricetin as Potent Inhibitors of Histidinol-Phosphate Aminotransferase in Drug-Resistant Acinetobacter baumannii.ACS Omega. 2025 Feb 20;10(8):7920-7936. doi: 10.1021/acsomega.4c08753. eCollection 2025 Mar 4. ACS Omega. 2025. PMID: 40060852 Free PMC article.
References
-
- Dawood FS, Chaves SS, Perez A, Reingold A, Meek J, Farley MM, Ryan P, Lynfield R, Morin C, Baumbach J, et al. Complications and associated bacterial coinfections among children hospitalized with seasonal or pandemic influenza, United States, 2003-2010. J Infect Dis. 2014;209(5):686–694. doi: 10.1093/infdis/jit473. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 81373141,81330082 and 81401312/National Natural Science Foundation of China
- 81621091/National Natural Science Foundation of China
- KJZD-EW-L15/the Intramural Special Grant for Influenza Virus Research from the Chinese Academy of Science
- JCYJ20160427151920801/the Shenzhen Science and Technology Research and Development Project
- 2016YFC1200800/the National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical

