Vaccine-Induced Protection from Homologous Tier 2 SHIV Challenge in Nonhuman Primates Depends on Serum-Neutralizing Antibody Titers
- PMID: 30552025
- PMCID: PMC6335502
- DOI: 10.1016/j.immuni.2018.11.011
Vaccine-Induced Protection from Homologous Tier 2 SHIV Challenge in Nonhuman Primates Depends on Serum-Neutralizing Antibody Titers
Abstract
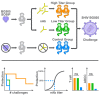
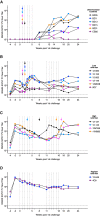
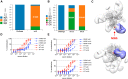
Passive administration of HIV neutralizing antibodies (nAbs) can protect macaques from hard-to-neutralize (tier 2) chimeric simian-human immunodeficiency virus (SHIV) challenge. However, conditions for nAb-mediated protection after vaccination have not been established. Here, we selected groups of 6 rhesus macaques with either high or low serum nAb titers from a total of 78 animals immunized with recombinant native-like (SOSIP) Env trimers. Repeat intrarectal challenge with homologous tier 2 SHIVBG505 led to rapid infection in unimmunized and low-titer animals. High-titer animals, however, demonstrated protection that was gradually lost as nAb titers waned over time. An autologous serum ID50 nAb titer of ∼1:500 afforded more than 90% protection from medium-dose SHIV infection. In contrast, antibody-dependent cellular cytotoxicity and T cell activity did not correlate with protection. Therefore, Env protein-based vaccination strategies can protect against hard-to-neutralize SHIV challenge in rhesus macaques by inducing tier 2 nAbs, provided appropriate neutralizing titers can be reached and maintained.
Keywords: ADCC; BG505; HIV vaccine; correlates of protection; neutralizing antibodies; non-human primates; tier 2 protection; vaccination.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Abrahams M.R., Anderson J.A., Giorgi E.E., Seoighe C., Mlisana K., Ping L.H., Athreya G.S., Treurnicht F.K., Keele B.F., Wood N., CAPRISA Acute Infection Study Team. Center for HIV-AIDS Vaccine Immunology Consortium Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J. Virol. 2009;83:3556–3567. - PMC - PubMed
-
- Alpert M.D., Heyer L.N., Williams D.E.J., Harvey J.D., Greenough T., Allhorn M., Evans D.T. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J. Virol. 2012;86:12039–12052. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI100663/AI/NIAID NIH HHS/United States
- R01 AI095098/AI/NIAID NIH HHS/United States
- UM1 AI126603/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- R01 AI121135/AI/NIAID NIH HHS/United States
- R01 AI098485/AI/NIAID NIH HHS/United States
- 352417/CIHR/Canada
- P30 AI036214/AI/NIAID NIH HHS/United States
- UM1 AI124377/AI/NIAID NIH HHS/United States
- R01 AI131331/AI/NIAID NIH HHS/United States
- R01 AI136621/AI/NIAID NIH HHS/United States
- P01 AI131251/AI/NIAID NIH HHS/United States
- F31 AI131873/AI/NIAID NIH HHS/United States
- P51 OD011106/OD/NIH HHS/United States
- R00 AI120851/AI/NIAID NIH HHS/United States
- U19 AI128751/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

