Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis
- PMID: 30552176
- PMCID: PMC6390132
- DOI: 10.1136/annrheumdis-2018-213336
Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis
Abstract
Objective: To assess the efficacy, safety, pharmacokinetics and pharmacodynamics of the anti-interleukin (IL)-1α/β dual variable domain immunoglobulin lutikizumab (ABT-981) in erosive hand osteoarthritis (HOA).
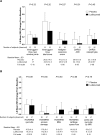
Methods: Patients with ≥1 erosive and ≥3 tender and/or swollen hand joints were randomised to placebo or lutikizumab 200 mg subcutaneously every 2 weeks for 24 weeks. The primary endpoint was change in Australian/Canadian Osteoarthritis Hand Index (AUSCAN) pain subdomain score from baseline to 16 weeks. At baseline and week 26, subjects had bilateral hand radiographs and MRI of the hand with the greatest number of baseline tender and/or swollen joints. Continuous endpoints were assessed using analysis of covariance models, with treatment and country as main factors and baseline measurements as covariates.
Results: Of 132 randomised subjects, 1 received no study drug and 110 completed the study (placebo, 61/67 (91%); lutikizumab, 49/64 (77%)). AUSCAN pain was not different among subjects treated with lutikizumab versus placebo at week 16 (least squares mean difference, 1.5 (95% CI -1.9 to 5.0)). Other clinical and imaging endpoints were not different between lutikizumab and placebo. Lutikizumab significantly decreased serum high-sensitivity C reactive protein levels, IL-1α and IL-1β levels, and blood neutrophils. Lutikizumab pharmacokinetics were consistent with phase I studies and not affected by antidrug antibodies. Injection site reactions and neutropaenia were more common in the lutikizumab group; discontinuations because of adverse events occurred more frequently with lutikizumab (4/64) versus placebo (1/67).
Conclusion: Despite adequate blockade of IL-1, lutikizumab did not improve pain or imaging outcomes in erosive HOA compared with placebo.
Trial registration: ClinicalTrials.gov NCT02384538.
Keywords: DMOADs (biologic); hand osteoarthritis; inflammation; interleukin-1.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AbbVie contributed to the design of the study and was involved in the collection, analysis and interpretation of the data, and in the writing, review and approval of the publication. MK has received grant/research support from Pfizer and been a consultant for AbbVie, GlaxoSmithKline, Merck and Levicept. CP is an employee of Spire Sciences, and is on the speakers' bureau for Amgen and Bristol-Myers Squibb. IKH has been a consultant for AbbVie. FK has no conflicts of interest to declare. RMF has received grant/research support from and has been a consultant for AbbVie. FB has been a consultant for AbbVie, Pfizer and Regeneron. DvdH has been a consultant for AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB; she is Director of Imaging Rheumatology. PB is a former employee of PAREXEL, which performed work for this study under contract to AbbVie. RW has been a consultant for AbbVie. SC, LW, WL, GL, YF, MS, JKM and MCL are employees of AbbVie and may own AbbVie stock and/or stock options. SF is a former employee of AbbVie and may own AbbVie stock and/or stock options.
Figures


Comment in
-
Can IL-1 be used as a target for osteoarthritis?Ann Rheum Dis. 2020 Jul;79(7):e88. doi: 10.1136/annrheumdis-2019-215513. Epub 2019 Apr 30. Ann Rheum Dis. 2020. PMID: 31040118 No abstract available.
-
Response to: 'Can IL-1 be used as a target for osteoarthritis?' by Cheng et al.Ann Rheum Dis. 2020 Jul;79(7):e89. doi: 10.1136/annrheumdis-2019-215612. Epub 2019 Jun 27. Ann Rheum Dis. 2020. PMID: 31248854 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

