Effects of Positive and Negative Expectations on Human Pain Perception Engage Separate But Interrelated and Dependently Regulated Cerebral Mechanisms
- PMID: 30552181
- PMCID: PMC6381241
- DOI: 10.1523/JNEUROSCI.2154-18.2018
Effects of Positive and Negative Expectations on Human Pain Perception Engage Separate But Interrelated and Dependently Regulated Cerebral Mechanisms
Abstract
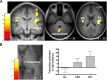
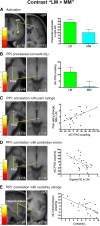
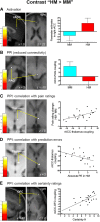
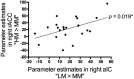
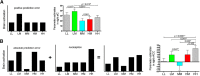
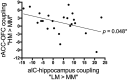
Expectations substantially influence pain perception, but the relationship between positive and negative expectations remains unclear. Recent evidence indicates that the integration between pain-related expectations and prediction errors is crucial for pain perception, which suggests that aversive prediction error-associated regions, such as the anterior insular cortex (aIC) and rostral anterior cingulate cortex (rACC), may play a pivotal role in expectation-induced pain modulation and help to delineate the relationship between positive and negative expectations. In a stimulus expectancy paradigm combining fMRI in healthy volunteers of both sexes, we found that, although positive and negative expectations respectively engaged the right aIC and right rACC to modulate pain, their associated activations and pain rating changes were significantly correlated. When positive and negative expectations modulated pain, the right aIC and rACC exhibited opposite coupling with periaqueductal gray (PAG) and the mismatch between actual and expected pain respectively modulated their coupling with PAG and thalamus across individuals. Participants' certainty about expectations predicted the extent of pain modulation, with positive expectations involving connectivity between aIC and hippocampus, a region regulating anxiety, and negative expectations engaging connectivity between rACC and lateral orbitofrontal cortex, a region reflecting outcome value and certainty. Interestingly, the strength of these certainty-related connectivities was also significantly associated between positive and negative expectations. These findings suggest that aversive prediction-error-related regions interact with pain-processing circuits to underlie stimulus expectancy effects on pain, with positive and negative expectations engaging dissociable but interrelated neural responses that are dependently regulated by individual certainty about expectations.SIGNIFICANCE STATEMENT Positive and negative expectations substantially influence pain perception, but their relationship remains unclear. Using fMRI in a stimulus expectancy paradigm, we found that, although positive and negative expectations engaged separate brain regions encoding the mismatch between actual and expected pain and involved opposite functional connectivities with the descending pain modulatory system, they produced significantly correlated pain rating changes and brain activation. Moreover, participants' certainty about expectations predicted the magnitude of both types of pain modulation, with the underlying functional connectivities significantly correlated between positive and negative expectations. These findings advance current understanding about cognitive modulation of pain, suggesting that both types of pain modulation engage different aversive prediction error signals but are dependently regulated by individual certainty about expectations.
Keywords: anterior cingulate cortex; expectation; functional magnetic resonance imaging; insula; pain; prediction error.
Copyright © 2019 the authors 0270-6474/19/391262-14$15.00/0.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
