Brettanomyces bruxellensis SSU1 Haplotypes Confer Different Levels of Sulfite Tolerance When Expressed in a Saccharomyces cerevisiae SSU1 Null Mutant
- PMID: 30552183
- PMCID: PMC6365814
- DOI: 10.1128/AEM.02429-18
Brettanomyces bruxellensis SSU1 Haplotypes Confer Different Levels of Sulfite Tolerance When Expressed in a Saccharomyces cerevisiae SSU1 Null Mutant
Abstract
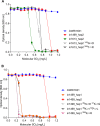
The addition of SO2 is practiced in the wine industry to mitigate the risk of microbial spoilage and to extend wine shelf-life. Generally, this strategy does not interfere with primary alcoholic fermentation, as wine strains of Saccharomyces cerevisiae exhibit significant SO2 tolerance, largely driven by the efflux pump Ssu1p. One of the key yeast species responsible for wine spoilage is Brettanomyces bruxellensis, which also exhibits strain-dependent SO2 tolerance, although this occurs via unknown mechanisms. To evaluate the factors responsible for the differential sulfite tolerance observed in B. bruxellensis strains, we employed a multifaceted approach to examine both expression and allelic differences in the BbSSU1 gene. Transcriptomic analysis following exposure to SO2 highlighted different inducible responses in two B. bruxellensis strains. It also revealed disproportionate transcription of one putative BbSSU1 haplotype in both genetic backgrounds. Here, we confirm the functionality of BbSSU1 by complementation of a null mutant in a S. cerevisiae wine strain. The expression of four distinct BbSSU1 haplotypes in the S. cerevisiae ΔSSU1 mutant revealed up to a 3-fold difference in conferred SO2 tolerance. Substitution of key amino acids distinguishing the encoded proteins was performed to evaluate their relative contribution to SO2 tolerance. Protein modeling of two haplotypes which differed in two amino acid residues suggested that these substitutions affect the binding of Ssu1p ligands near the channel opening. Taken together, preferential transcription of a BbSSU1 allele that encodes a more efficient Ssu1p transporter may represent one mechanism that contributes to differences in sulfite tolerances between B. bruxellensis strains.IMPORTANCEBrettanomyces bruxellensis is one of the most important wine spoilage microorganisms, with the use of sulfite being the major method to control spoilage. However, this species displays a wide intraspecies distribution in sulfite tolerance, with some strains capable of tolerating high concentrations of SO2, with relatively high concentrations of this antimicrobial needed for their control. Although SO2 tolerance has been studied in several organisms and particularly in S. cerevisiae, little is known about the mechanisms that confer SO2 tolerance in B. bruxellensis Here, we confirmed the functionality of the sulfite efflux pump encoded by BbSSU1 and determined the efficiencies of four different BbSSU1 haplotypes. Gene expression analysis showed greater expression of the haplotype conferring greater SO2 tolerance. Our results suggest that a combination of BbSSU1 haplotype efficiency, copy number, and haplotype expression levels likely contributes to the diverse SO2 tolerances observed for different B. bruxellensis strains.
Keywords: SO2; Ssu1p; allele specific expression; transcriptome; wine; yeast.
Copyright © 2019 American Society for Microbiology.
Figures






References
-
- Claussen NH. 1904. On a method for the application of Hansen's pure yeast system in the manufacturing of well‐conditioned English stock beers. J Inst Brewing 10:308–331. doi: 10.1002/j.2050-0416.1904.tb04656.x. - DOI
-
- de Souza Liberal A, Basílio A, do Monte Resende A, Brasileiro B, Silva‐Filho D, De Morais J, Simões D, De Morais M. 2007. Identification of Dekkera bruxellensis as a major contaminant yeast in continuous fuel ethanol fermentation. J Appl Microbiol 102:538–547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

