Galactose Impacts the Size and Intracellular Composition of the Asaccharolytic Oral Pathobiont Porphyromonas gingivalis
- PMID: 30552185
- PMCID: PMC6365826
- DOI: 10.1128/AEM.02268-18
Galactose Impacts the Size and Intracellular Composition of the Asaccharolytic Oral Pathobiont Porphyromonas gingivalis
Abstract
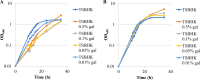
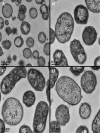
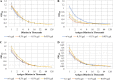
The asaccharolytic anaerobe Porphyromonas gingivalis metabolizes proteins it encounters in the periodontal pocket, including host-derived glycoproteins such as mucins and immunoglobulins. Often, these proteins are protected by a diverse array of carbohydrates tethered to the polypeptide chain via glycolytic bonds, and P. gingivalis produces enzymes capable of liberating these carbohydrates, exposing the proteinaceous core. In this study, we investigated the effect of individual monosaccharides, including galactose, l-fucose, mannose, and glucose, on the growth and physiology of P. gingivalis Of the carbohydrates tested, only galactose noticeably altered the density of the bacterial culture, and we observed that cultures grown with galactose reached significantly higher densities during stationary phase. Importantly, electron micrographs and plating of P. gingivalis in stationary phase demonstrated that the presence of galactose did not increase cell numbers; instead, the higher densities resulted from the expansion of individual cells which contained large intracellular granules. Initial attempts to characterize these granules revealed only a subtle increase in soluble carbohydrates, suggesting they are likely not composed of stored carbohydrate. Also, an analysis of major surface polysaccharides via an enzyme-linked immunosorbent assay (ELISA) did not reveal significant differences between cells grown with or without galactose. Finally, an initial investigation of the transcriptional changes elicited by galactose in late exponential phase suggested that genes important for cell shape and for the general stress response may play roles in this phenomenon. Overall, galactose, a monosaccharide commonly present on the surfaces of host proteins, substantially alters the physiology of P. gingivalis via the production of large, currently undefined, intracellular granules.IMPORTANCE Environmental perturbations are central to the ability of pathobionts, such as Porphyromonas gingivalis, to promote the development of diseased sites. In the case of periodontal disease, increased local pH, a shift to anaerobic surroundings, and the accumulation of Gram-negative anaerobes at the expense of Gram-positive cocci are known ecological fluctuations prominently associated with progression toward disease. Importantly, in contrast, the alterations to subgingival food webs in disease sites remain poorly characterized. We hypothesized that given the dramatic shift in community structure during disease, it is possible that free carbohydrates, which would typically be readily metabolized by Gram-positive cocci after cleavage from glycoproteins, may increase in concentration locally and thereby affect the physiological state of the subgingival microbiota. In this study, we explored the impact of free monosaccharides on P. gingivalis to gain deeper insight into the effect of dysbiotic conditions on the growth and physiology of this periodontal pathogen.
Keywords: microbial physiology; stress response.
Copyright © 2019 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

