Ex vivo conditioning with IL-12 protects tumor-infiltrating CD8+ T cells from negative regulation by local IFN-γ
- PMID: 30552459
- PMCID: PMC6428620
- DOI: 10.1007/s00262-018-2280-3
Ex vivo conditioning with IL-12 protects tumor-infiltrating CD8+ T cells from negative regulation by local IFN-γ
Abstract
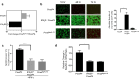
Optimal ex vivo expansion protocols for adoptive cell therapy (ACT) must yield T cells able to effectively home to tumors and survive the inhospitable conditions of the tumor microenvironment (TME), while simultaneously exerting persistent anti-tumor effector functions. Our previous work has shown that ex vivo activation in the presence of IL-12 can induce optimal expansion of murine CD8+ T cells, thus resulting in significant tumor regression after ACT mostly via sustained secretion of IFN-γ. In this report, we further elucidate the mechanism of this potency, showing that IL-12 additionally counteracts the negative regulatory effects of autocrine IFN-γ. IL-12 not only downregulates PD-1 expression by T cells, thus minimizing the effects of IFN-γ-induced PD-L1 upregulation by tumor stromal cells, but also inhibits IFNγR2 expression, thereby protecting T cells from IFN-γ-induced cell death. Thus, the enhanced anti-tumor activity of CD8+ T cells expanded ex vivo in the presence of IL-12 is due not only to the ability of IL-12-stimulated cells to secrete sustained levels of IFN-γ, but also to the additional capacity of IL-12 to counter the negative regulatory effects of autocrine IFN-γ.
Keywords: Adoptive T cells transfer; Melanoma/skin cancers; Models of host–tumor interactions; PD-1; Tumor microenvironment; Tumor promotion and progression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4 + and CD8+ T cells. J Immunol. 1999;162(6):3256–3262. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

