Pharmacokinetics of Prolonged-Release Once-Daily Formulations of Tacrolimus in De Novo Kidney Transplant Recipients: A Randomized, Parallel-Group, Open-Label, Multicenter Study
- PMID: 30552587
- PMCID: PMC6824349
- DOI: 10.1007/s12325-018-0855-1
Pharmacokinetics of Prolonged-Release Once-Daily Formulations of Tacrolimus in De Novo Kidney Transplant Recipients: A Randomized, Parallel-Group, Open-Label, Multicenter Study
Abstract
Introduction: Different prolonged-release formulations of tacrolimus are available. To date, the pharmacokinetic (PK) profile of LCP-tacrolimus (LCPT; Envarsus®) has not been compared with PR-Tac (Advagraf®) in de novo kidney transplant recipients. These profiles will guide clinical recommendations for the initiation and dose titration strategies of once-daily tacrolimus formulations.
Methods: This randomized, parallel-group, open-label, multicenter PK study randomized 75 de novo, adult, white kidney transplant recipients to LCPT 0.17 mg/kg/day (n = 37) or PR-Tac 0.20 mg/kg/day (n = 38) for 4 weeks. Dose adjustments were permitted to target a pre-defined therapeutic range based on measured trough blood concentrations.
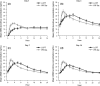
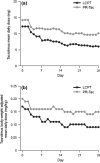
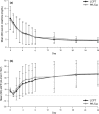
Results: PK analysis (days 1, 3, 7 and 14) included 68 patients (LCPT, n = 33; PR-Tac, n = 35). Similar proportions of patients were within the pre-defined therapeutic tacrolimus trough blood concentration range, with < 12% in each group having below-target trough levels over the study period. LCPT demonstrated ~ 30% greater relative bioavailability [LCPT/PR-Tac adjusted geometric mean ratio: day 3, 1.32 (p = 0.007); day 7, 1.25 (p = 0.051); day 14, 1.43 (p = 0.002)] and ~ 30% lower peak-to-trough percentage fluctuation of blood concentration [LCPT/PR-Tac adjusted geometric mean ratio: day 3, 0.70 (p < 0.001); day 7, 0.68 (p < 0.001); day 14, 0.73 (p = 0.004)] in addition to longer time to maximum blood concentration (tmax), lower maximum concentration (Cmax) and a consistently lower daily dose (~ 40% dose reduction with LCPT vs. PR-Tac by day 28). Safety profiles were similar.
Conclusion: In de novo kidney transplant recipients, prolonged-release formulations of tacrolimus can reach therapeutic concentrations in the immediate post-transplant period. LCPT has greater relative bioavailability and lower peak-to-trough fluctuation compared with PR-Tac.
Trial registration: Registered at ClinicalTrials.gov; study number NCT02500212.
Funding: Chiesi Farmaceutici S.p.A.
Keywords: Advagraf; De novo kidney transplantation; Envarsus; LCPT; PR Tac; Pharmacokinetics; Tacrolimus.
Figures



References
-
- Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. - PubMed
-
- Food and Drug Administration. Draft guidance on tacrolimus june 2016.
-
- Krzyzowska K, Kolonko A, Giza P, Chudek J, Wiecek A. No significant influence of reduced initial tacrolimus dose on risk of underdosing and early graft function in older and overweight kidney transplant recipients. Transpl Proc. 2018;50(6):1755–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

