Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma
- PMID: 30553841
- PMCID: PMC10878126
- DOI: 10.1016/j.jhep.2018.12.004
Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma
Abstract
Background & aims: In cholangiocarcinoma, early metastatic spread via lymphatic vessels often precludes curative therapies. Cholangiocarcinoma invasiveness is fostered by an extensive stromal reaction, enriched in cancer-associated fibroblasts (CAFs) and lymphatic endothelial cells (LECs). Cholangiocarcinoma cells recruit and activate CAFs by secreting PDGF-D. Herein, we investigated the role of PDGF-D and liver myofibroblasts in promoting lymphangiogenesis in cholangiocarcinoma.
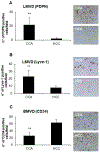
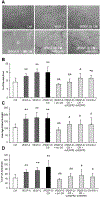
Methods: Human cholangiocarcinoma specimens were immunostained for podoplanin (LEC marker), α-SMA (CAF marker), VEGF-A, VEGF-C, and their cognate receptors (VEGFR2, VEGFR3). VEGF-A and VEGF-C secretion was evaluated in human fibroblasts obtained from primary sclerosing cholangitis explants. Using human LECs incubated with conditioned medium from PDGF-D-stimulated fibroblasts we assessed migration, 3D vascular assembly, transendothelial electric resistance and transendothelial migration of cholangiocarcinoma cells (EGI-1). We then studied the effects of selective CAF depletion induced by the BH3 mimetic navitoclax on LEC density and lymph node metastases in vivo.
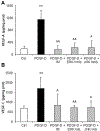
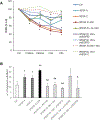
Results: In cholangiocarcinoma specimens, CAFs and LECs were closely adjacent. CAFs expressed VEGF-A and VEGF-C, while LECs expressed VEGFR2 and VEGFR3. Upon PDGF-D stimulation, fibroblasts secreted increased levels of VEGF-C and VEGF-A. Fibroblasts, stimulated by PDGF-D induced LEC recruitment and 3D assembly, increased LEC monolayer permeability, and promoted transendothelial EGI-1 migration. These effects were all suppressed by the PDGFRβ inhibitor, imatinib. In the rat model of cholangiocarcinoma, navitoclax-induced CAF depletion, markedly reduced lymphatic vascularization and reduced lymph node metastases.
Conclusion: PDGF-D stimulates VEGF-C and VEGF-A production by fibroblasts, resulting in expansion of the lymphatic vasculature and tumor cell intravasation. This critical process in the early metastasis of cholangiocarcinoma may be blocked by inducing CAF apoptosis or by inhibiting the PDGF-D-induced axis.
Lay summary: Cholangiocarcinoma is a highly malignant cancer affecting the biliary tree, which is characterized by a rich stromal reaction involving a dense population of cancer-associated fibroblasts that promote early metastatic spread. Herein, we show that cholangiocarcinoma-derived PDGF-D stimulates fibroblasts to secrete vascular growth factors. Thus, targeting fibroblasts or PDGF-D-induced signals may represent an effective tool to block tumor-associated lymphangiogenesis and reduce the invasiveness of cholangiocarcinoma.
Keywords: Cholangiocytes; Lymphatic endothelial cells; Tumor reactive stroma; VEGF-C; VEGFR3.
Copyright © 2018 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



References
-
- Banales JM, Cardinale V, Carpino V, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–280. - PubMed
-
- Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gessner R, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol 2010;105:1123–1132. - PubMed
-
- Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival Outcomes and Prognostic Factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg 2014;18:562–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

