Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial
- PMID: 30554748
- PMCID: PMC6293968
- DOI: 10.1016/S2214-109X(18)30444-3
Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial
Abstract
Background: In southeast Asia, antibiotic prescription in febrile patients attending primary care is common, and a probable contributor to the high burden of antimicrobial resistance. The objective of this trial was to explore whether C-reactive protein (CRP) testing at point of care could rationalise antibiotic prescription in primary care, comparing two proposed thresholds to classify CRP concentrations as low or high to guide antibiotic treatment.
Methods: We did a multicentre, open-label, randomised, controlled trial in participants aged at least 1 year with a documented fever or a chief complaint of fever (regardless of previous antibiotic intake and comorbidities other than malignancies) recruited from six public primary care units in Thailand and three primary care clinics and one outpatient department in Myanmar. Individuals were randomly assigned using a computer-based randomisation system at a ratio of 1:1:1 to either the control group or one of two CRP testing groups, which used thresholds of 20 mg/L (group A) or 40 mg/L CRP (group B) to guide antibiotic prescription. Health-care providers were masked to allocation between the two intervention groups but not to the control group. The primary outcome was the prescription of any antibiotic from day 0 to day 5 and the proportion of patients who were prescribed an antibiotic when CRP concentrations were above and below the 20 mg/L or 40 mg/L thresholds. The primary outcome was analysed in the intention-to-treat and per-protocol populations. The trial is registered with ClinicalTrials.gov, number NCT02758821, and is now completed.
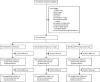
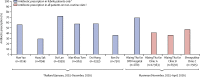
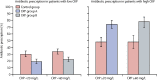
Findings: Between June 8, 2016, and Aug 25, 2017, we recruited 2410 patients, of whom 803 patients were randomly assigned to CRP group A, 800 to CRP group B, and 807 to the control group. 598 patients in CRP group A, 593 in CRP group B, and 767 in the control group had follow-up data for both day 5 and day 14 and had been prescribed antibiotics (or not) in accordance with test results (per-protocol population). During the trial, 318 (39%) of 807 patients in the control group were prescribed an antibiotic by day 5, compared with 290 (36%) of 803 patients in CRP group A and 275 (34%) of 800 in CRP group B. The adjusted odds ratio (aOR) of 0·80 (95% CI 0·65-0·98) and risk difference of -5·0 percentage points (95% CI -9·7 to -0·3) between group B and the control group were significant, although lower than anticipated, whereas the reduction in prescribing in group A compared with the control group was not significant (aOR 0·86 [0·70-1·06]; risk difference -3·3 percentage points [-8·0 to 1·4]). Patients with high CRP concentrations in both intervention groups were more likely to be prescribed an antibiotic than in the control group (CRP ≥20 mg/L: group A vs control group, p<0·0001; CRP ≥40 mg/L: group B vs control group, p<0·0001), and those with low CRP concentrations were more likely to have an antibiotic withheld (CRP <20 mg/L: group A vs control group, p<0·0001; CRP <40 mg/L: group B vs control group, p<0·0001). 24 serious adverse events were recorded, consisting of 23 hospital admissions and one death, which occurred in CRP group A. Only one serious adverse event was thought to be possibly related to the study (a hospital admission in CRP group A).
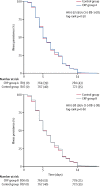
Interpretation: In febrile patients attending primary care, testing for CRP at point of care with a threshold of 40 mg/L resulted in a modest but significant reduction in antibiotic prescribing, with patients with high CRP being more likely to be prescribed an antibiotic, and no evidence of a difference in clinical outcomes. This study extends the evidence base from lower-income settings supporting the use of CRP tests to rationalise antibiotic use in primary care patients with an acute febrile illness. A key limitation of this study is the individual rather than cluster randomised study design which might have resulted in contamination between the study groups, reducing the effect size of the intervention.
Funding: Wellcome Trust Institutional Strategic Support Fund grant (105605/Z/14/Z) and Foundation for Innovative New Diagnostics (FIND) funding from the Australian Government.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Biomarkers to improve rational antibiotic use in low-resource settings.Lancet Glob Health. 2019 Jan;7(1):e14-e15. doi: 10.1016/S2214-109X(18)30497-2. Lancet Glob Health. 2019. PMID: 30554750 No abstract available.
-
Point-of-care C-reactive protein testing and antibiotic prescribing.Lancet Glob Health. 2021 Jan;9(1):e16. doi: 10.1016/S2214-109X(20)30451-4. Lancet Glob Health. 2021. PMID: 33338451 No abstract available.
References
-
- Hongoro C, McPake B. How to bridge the gap in human resources for health. Lancet. 2004;364:1451–1456. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

