Tissue iron is negatively correlated with TERC or TERT mRNA expression: A heterochronic parabiosis study in mice
- PMID: 30555055
- PMCID: PMC6326661
- DOI: 10.18632/aging.101676
Tissue iron is negatively correlated with TERC or TERT mRNA expression: A heterochronic parabiosis study in mice
Abstract
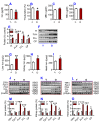
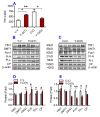
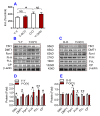
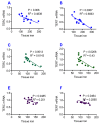
To test the hypothesis that iron accumulation in tissues with age is a key harmful factor for the development of aging, we established heterochronic parabiosis-pairings and investigated changes in serum iron, the expression of major iron transport proteins and iron contents, as well as telomerase reverse transcriptase (TERT), telomerase RNA component (TERC), and telomere length in the liver, kidney and heart of Y-O(O) (old pairing with young), Y-O(Y) (young pairing with old), O-O (pairings between two old) and Y-Y (pairings between two young) mice. We demonstrated that the reduced serum iron, increased iron and reduced expression of TERT and TERC in the tissues of aged mice are reversible by exposure to a younger mouse's circulation. All of these measurements in young mice are reversible by exposure to an older mouse's circulation. Correlation analysis showed that tissue iron is negatively correlated with TERT and TERC expression in the liver, kidney and heart of parabiotic mice. These findings provide new evidence for the key role of iron in aging and also imply the existence of rejuvenating factors in young serum with an anti-ageing role that act by reversing the impaired activity of iron metabolism in old mice.
Keywords: heterochronic parabiosis; iron homeostasis; liver, kidney and heart of mice; telomere and telomerase; young and old mice.
Conflict of interest statement
Figures



References
-
- Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, et al.. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013; 153:828–39. 10.1016/j.cell.2013.04.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

