The oral selective oestrogen receptor degrader (SERD) AZD9496 is comparable to fulvestrant in antagonising ER and circumventing endocrine resistance
- PMID: 30555156
- PMCID: PMC6353941
- DOI: 10.1038/s41416-018-0354-9
The oral selective oestrogen receptor degrader (SERD) AZD9496 is comparable to fulvestrant in antagonising ER and circumventing endocrine resistance
Abstract
Background: The oestrogen receptor (ER) is an important therapeutic target in ER-positive (ER+) breast cancer. The selective ER degrader (SERD), fulvestrant, is effective in patients with metastatic breast cancer, but its intramuscular route of administration and low bioavailability are major clinical limitations.
Methods: Here, we studied the pharmacology of a new oral SERD, AZD9496, in a panel of in vitro and in vivo endocrine-sensitive and -resistant breast cancer models.
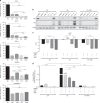
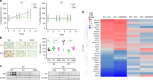
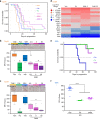
Results: In endocrine-sensitive models, AZD9496 inhibited cell growth and blocked ER activity in the presence or absence of oestrogen. In vivo, in the presence of oestrogen, short-term AZD9496 treatment, like fulvestrant, resulted in tumour growth inhibition and reduced expression of ER-dependent genes. AZD9496 inhibited cell growth in oestrogen deprivation-resistant and tamoxifen-resistant cell lines and xenograft models that retain ER expression. AZD9496 effectively reduced ER levels and ER-induced transcription. Expression analysis of short-term treated tumours showed that AZD9496 potently inhibited classic oestrogen-induced gene transcription, while simultaneously increasing expression of genes negatively regulated by ER, including genes potentially involved in escape pathways of endocrine resistance.
Conclusions: These data suggest that AZD9496 is a potent anti-oestrogen that antagonises and degrades ER with anti-tumour activity in both endocrine-sensitive and endocrine-resistant models.
Conflict of interest statement
R.S. has received research funding (to institution) from AstraZeneca, Gilead, PUMA, and GlaxoSmithKline (GSK). She is a consulting/advisory committee member for Macrogenics, and Eli Lilly. C.K.O. has received research funding from AstraZeneca and GSK. He has been a member of advisory boards for Pfizer, Nanostring, Genentech, Tolmar Pharmaceuticals, and AstraZeneca. He is also a member of a DMC for Eli Lilly. M.F.R. has received research funding (to institution) from Pfizer and GSK. He is a consulting member for Genentech, Novartis, Daiichi Sankyo, and Macrogenics. R.J. received research funding from Pfizer. O.D. is an AstraZeneca employee. H.W., M.P., and H.B. are former AstraZeneca employees.
Figures

References
-
- van Kruchten M, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5:72–81. doi: 10.1158/2159-8290.CD-14-0697. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

