Pathophysiology of NSAID-Associated Intestinal Lesions in the Rat: Luminal Bacteria and Mucosal Inflammation as Targets for Prevention
- PMID: 30555323
- PMCID: PMC6281992
- DOI: 10.3389/fphar.2018.01340
Pathophysiology of NSAID-Associated Intestinal Lesions in the Rat: Luminal Bacteria and Mucosal Inflammation as Targets for Prevention
Abstract
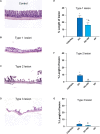
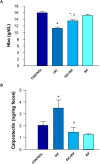
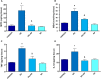
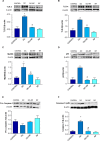
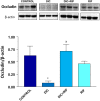
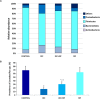
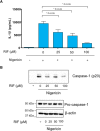
Non-steroidal anti-inflammatory drugs (NSAIDs) can damage the small intestine, mainly through an involvement of enteric bacteria. This study examined the pathophysiology of NSAID-associated intestinal lesions in a rat model of diclofenac-enteropathy and evaluated the effect of rifaximin on small bowel damage. Enteropathy was induced in 40-week old male rats by intragastric diclofenac (4 mg/kg BID, 14 days). Rifaximin (delayed release formulation) was administered (50 mg/kg BID) 1 h before the NSAID. At the end of treatments, parameters dealing with ileal damage, inflammation, barrier integrity, microbiota composition, and TLR-NF-κB-inflammasome pathway were evaluated. In addition, the modulating effect of rifaximin on NLRP3 inflammasome was tested in an in vitro cell system. Diclofenac induced intestinal damage and inflammation, triggering an increase in tissue concentrations of tumor necrosis factor and interleukin-1β, higher expression of TLR-2 and TLR-4, MyD88, NF-κB and activation of caspase-1. In addition, the NSAID decreased ileal occludin expression and provoked a shift of bacterial phyla toward an increase in Proteobacteria and Bacteroidetes abundance. All these changes were counterbalanced by rifaximin co-administration. This drug was also capable of increasing the proportion of Lactobacilli, a genus depleted by the NSAID. In LPS-primed THP-1 cells stimulated by nigericin (a model to study the NLRP3 inflammasome), rifaximin reduced IL-1β production in a concentration-dependent fashion, this effect being associated with inhibition of the up-stream caspase-1 activation. In conclusion, diclofenac induced ileal mucosal lesions, driving inflammatory pathways and microbiota changes. In conclusion, rifaximin prevents diclofenac-induced enteropathy through both anti-bacterial and anti-inflammatory activities.
Keywords: enteroprotection; intestinal bleeding; intestinal damage; microbiota; non-steroidal anti-inflammatory drugs; rifaximin.
Figures
Similar articles
-
Use of Saccharomyces boulardii CNCM I-745 as therapeutic strategy for prevention of nonsteroidal anti-inflammatory drug-induced intestinal injury.Br J Pharmacol. 2023 Dec;180(24):3215-3233. doi: 10.1111/bph.16200. Epub 2023 Aug 27. Br J Pharmacol. 2023. PMID: 37519261
-
Small bowel protection against NSAID-injury in rats: Effect of rifaximin, a poorly absorbed, GI targeted, antibiotic.Pharmacol Res. 2016 Feb;104:186-96. doi: 10.1016/j.phrs.2015.12.031. Epub 2015 Dec 30. Pharmacol Res. 2016. PMID: 26747402
-
Protective effects of the combination Bifidobacterium longum plus lactoferrin against NSAID-induced enteropathy.Nutrition. 2020 Feb;70:110583. doi: 10.1016/j.nut.2019.110583. Epub 2019 Sep 12. Nutrition. 2020. PMID: 31739175
-
Microbiota Plays a Key Role in Non-Steroidal Anti-Inflammatory Drug-Induced Small Intestinal Damage.Digestion. 2017;95(1):22-28. doi: 10.1159/000452356. Epub 2017 Jan 5. Digestion. 2017. PMID: 28052268 Review.
-
Microbial flora in NSAID-induced intestinal damage: a role for antibiotics?Digestion. 2006;73 Suppl 1:136-50. doi: 10.1159/000089789. Epub 2006 Feb 8. Digestion. 2006. PMID: 16498262 Review.
Cited by
-
Drug-Induced Small Bowel Injury: a Challenging and Often Forgotten Clinical Condition.Curr Gastroenterol Rep. 2019 Nov 13;21(11):55. doi: 10.1007/s11894-019-0726-1. Curr Gastroenterol Rep. 2019. PMID: 31720893 Review.
-
Lack of Small Intestinal Dysbiosis Following Long-Term Selective Inhibition of Cyclooxygenase-2 by Rofecoxib in the Rat.Cells. 2019 Mar 15;8(3):251. doi: 10.3390/cells8030251. Cells. 2019. PMID: 30884758 Free PMC article.
-
The Combined Effect of Western Diet Consumption and Diclofenac Administration Alters the Gut Microbiota and Promotes Anastomotic Leakage in the Distal Colon.Biomedicines. 2024 Sep 24;12(10):2170. doi: 10.3390/biomedicines12102170. Biomedicines. 2024. PMID: 39457483 Free PMC article.
-
Modulation of the gut microbiota and the microbial-produced gut metabolites by diclofenac exposure and selenium supplementation.Environ Sci Pollut Res Int. 2025 Jun;32(28):16945-16957. doi: 10.1007/s11356-025-36233-6. Epub 2025 Mar 18. Environ Sci Pollut Res Int. 2025. PMID: 40102351 Free PMC article.
-
Gut Microbiota in NSAID Enteropathy: New Insights From Inside.Front Cell Infect Microbiol. 2021 Jul 6;11:679396. doi: 10.3389/fcimb.2021.679396. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34295835 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

