IFN-β Plays Both Pro- and Anti-inflammatory Roles in the Rat Cardiac Fibroblast Through Differential STAT Protein Activation
- PMID: 30555324
- PMCID: PMC6280699
- DOI: 10.3389/fphar.2018.01368
IFN-β Plays Both Pro- and Anti-inflammatory Roles in the Rat Cardiac Fibroblast Through Differential STAT Protein Activation
Abstract
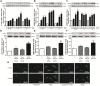
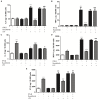
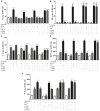
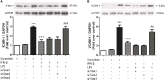
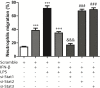
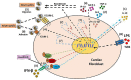
Cardiac fibroblasts (CFs) contribute to theinflammatory response to tissue damage, secreting both pro- and anti-inflammatory cytokines and chemokines. Interferon beta (IFN-β) induces the phosphorylation of signal transducer and activator of transcription (STAT) proteins through the activation of its own receptor, modulating the secretion of cytokines and chemokines which regulate inflammation. However, the role of IFN-β and STAT proteins in modulating the inflammatory response of CF remains unknown. CF were isolated from adult male rats and subsequently stimulated with IFN-β to evaluate the participation of STAT proteins in secreting chemokines, cytokines, cell adhesion proteins expression and in their capacity to recruit neutrophils. In addition, in CF in which the TRL4 receptor was pre-activated, the effect of INF-β on the aforementioned responses was also evaluated. Cardiac fibroblasts stimulation with IFN-β showed an increase in STAT1, STAT2, and STAT3 phosphorylation. IFN-β stimulation through STAT1 activation increased proinflammatory chemokines MCP-1 and IP-10 secretion, whereas IFN-β induced activation of STAT3 increased cytokine secretion of anti-inflammatory IL-10. Moreover, in TLR4-activated CF, IFN-β through STAT2 and/or STAT3, produced an anti-inflammatory effect, reducing pro-IL-1β, TNF-α, IL-6, MCP-1, and IP-10 secretion; and decreasing neutrophil recruitment by decreasing ICAM-1 and VCAM-1 expression. Altogether, our results indicate that IFN-β exerts both pro-inflammatory and anti-inflammatory effects in non-stimulated CF, through differential activation of STAT proteins. When CF were previously treated with an inflammatory agent such as TLR-4 activation, IFN-β effects were predominantly anti-inflammatory.
Keywords: IFN-β (interferon β); STAT; anti-infammatory; cardiac fibroblast; proinflammatory.
Figures


References
-
- Boza P., Ayala P., Vivar R., Humeres C., Tapia Cáceres F., Muñoz C., et al. (2016). Expression and function of toll-like receptor 4 and inflammasomes in cardiac fibroblasts and myofibroblasts: IL-1β synthesis, secretion, and degradation. Mol. Immunol. 74 96–105. 10.1016/j.molimm.2016.05.001 - DOI - PubMed
-
- Coclet-Ninin J., Dayer J. M., Burger D. (1997). Interferon-beta not only inhibits interleukin-1beta and tumor necrosis factor-alpha but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur. Cytokine Netw. 8 345–349. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

