The Nociceptin Receptor (NOP) Agonist AT-312 Blocks Acquisition of Morphine- and Cocaine-Induced Conditioned Place Preference in Mice
- PMID: 30555362
- PMCID: PMC6281746
- DOI: 10.3389/fpsyt.2018.00638
The Nociceptin Receptor (NOP) Agonist AT-312 Blocks Acquisition of Morphine- and Cocaine-Induced Conditioned Place Preference in Mice
Abstract
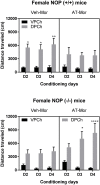
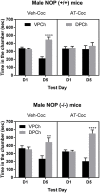
Treatment of drug addiction remains an unmet medical need due to the dearth of approved pharmacotherapies. There are no approved treatments for cocaine addiction, whereas the current opioid crisis has revealed the stark reality of the limited options to treat prescription and illicit opioid abuse. Preclinical studies in rodents and nonhuman primates have shown that orphanin FQ/nociceptin (N/OFQ), the endogenous ligand for the nociceptin opioid receptor (NOP) reduces the rewarding effects of several abused substances, including opioids, psychostimulants and alcohol. A few nonpeptide small-molecule NOP agonists have also shown efficacy in attenuating the rewarding effects of various abused drugs. We previously demonstrated that a high affinity small-molecule NOP agonist AT-312 selectively reduced the rewarding effects of ethanol in the conditioned place preference paradigm in mice. In the present study, we examined if AT-312 (3 mg/kg, i.p. or s.c. respectively), would alter the rewarding action of morphine (7.5 mg/kg, s.c.) or cocaine (15 mg/kg, i.p.). The effect of AT-312 on morphine- and cocaine-induced motor stimulation was also assessed on the conditioning days. The role of the NOP receptor in the effects of AT-312 was further confirmed by conducting the place conditioning experiments in NOP knockout mice and compared to their wild-type controls. Our results showed that AT-312 significantly reduced the acquisition of morphine and cocaine CPP in wild-type mice but not in mice lacking NOP receptors. AT-312 also suppressed morphine-induced and completely abolished cocaine-induced motor stimulation in NOP wild-type mice, but not in NOP knockout mice. These results show that small-molecule NOP receptor agonists have promising efficacy for attenuating the rewarding effects of morphine and cocaine, and may have potential as pharmacotherapy for opioid and psychostimulant addiction or for treating polydrug addiction.
Keywords: AT-312; NOP agonist; NOP receptor knockout; addiction pharmacotherapy; cocaine; conditioned place preference; morphine; polydrug addiction.
Figures


References
-
- Kuhar MJ, Ritz MC, Sharkey J. Cocaine receptors on dopamine transporters mediate cocaine-reinforced behavior. NIDA Res Monogr. (1988) 88:14–22. - PubMed

