Tissue Resident CD8 Memory T Cell Responses in Cancer and Autoimmunity
- PMID: 30555481
- PMCID: PMC6281983
- DOI: 10.3389/fimmu.2018.02810
Tissue Resident CD8 Memory T Cell Responses in Cancer and Autoimmunity
Abstract
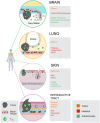
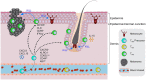
Resident memory (TRM) cells are a distinct tissue-localized T cell lineage that is crucial for protective immunity in peripheral tissues. While a great deal of effort has focused on defining their role in immunity to infections, studies now reveal TRM cells as a vital component of the host immune response to cancer. Characterized by cell-surface molecules including CD103, CD69, and CD49a, TRM-like tumor-infiltrating lymphocytes (TILs) can be found in a wide range of human cancers, where they portend improved prognosis. Recent studies in mouse tumor models have shown that TRM cells are induced by cancer vaccines delivered in peripheral tissue sites, or by the depletion of regulatory T cells. Such tumor-specific TRM cells are recognized as both necessary and sufficient for long-lived protection against tumors in peripheral tissue locations. TRM responses against tumor/self-antigens can concurrently result in the development of pathogenic TRM responses to self, with a growing number of autoimmune diseases and inflammatory pathologies being attributed to TRM responses. This review will recount the path to discovering the importance of resident memory CD8 T cells as they pertain to cancer immunity. In addition to highlighting key studies that directly implicate TRM cells in anti-tumor immunity, we will highlight earlier work that implicitly suggested their importance. Informed by studies in infectious disease models, and instructed by a clear role for TRM cells in autoimmunity, we will discuss strategies for therapeutically promoting TRM responses in settings where they don't naturally occur.
Keywords: CD103; TCM; TIL; TRM; biomarker; immunotherapy; melanoma; vitiligo.
Figures
References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. (2018) 8:1069–86. 10.1158/2159-8290.CD-18-0367 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

