Dual-Isotope SPECT/CT Imaging of the Tuberculosis Subunit Vaccine H56/CAF01: Induction of Strong Systemic and Mucosal IgA and T-Cell Responses in Mice Upon Subcutaneous Prime and Intrapulmonary Boost Immunization
- PMID: 30555488
- PMCID: PMC6284049
- DOI: 10.3389/fimmu.2018.02825
Dual-Isotope SPECT/CT Imaging of the Tuberculosis Subunit Vaccine H56/CAF01: Induction of Strong Systemic and Mucosal IgA and T-Cell Responses in Mice Upon Subcutaneous Prime and Intrapulmonary Boost Immunization
Abstract
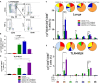
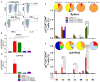
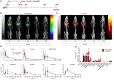
Pulmonary tuberculosis (TB), which is caused by Mycobacterium tuberculosis (Mtb), remains a global pandemic, despite the widespread use of the parenteral live attenuated Bacillus Calmette-Guérin (BCG) vaccine during the past decades. Mucosal administration of next generation TB vaccines has great potential, but developing a safe and efficacious mucosal vaccine is challenging. Hence, understanding the in vivo biodistribution and pharmacokinetics of mucosal vaccines is essential for shaping the desired immune response and for optimal spatiotemporal targeting of the appropriate effector cells in the lungs. A subunit vaccine consisting of the fusion antigen H56 (Ag85B-ESAT-6-Rv2660) and the liposome-based cationic adjuvant formulation (CAF01) confers efficient protection in preclinical animal models. In this study, we devise a novel immunization strategy for the H56/CAF01 vaccine, which comply with the intrapulmonary (i.pulmon.) route of immunization. We also describe a novel dual-isotope (111In/67Ga) radiolabeling approach, which enables simultaneous non-invasive and longitudinal SPECT/CT imaging and quantification of H56 and CAF01 upon parenteral prime and/or i.pulmon. boost immunization. Our results demonstrate that the vaccine is distributed evenly in the lungs, and there are pronounced differences in the pharmacokinetics of H56 and CAF01. We provide convincing evidence that the H56/CAF01 vaccine is not only well-tolerated when administered to the respiratory tract, but it also induces strong lung mucosal and systemic IgA and polyfunctional Th1 and Th17 responses after parenteral prime and i.pulmon. boost immunization. The study furthermore evaluate the application of SPECT/CT imaging for the investigation of vaccine biodistribution after parenteral and i.pulmon. immunization of mice.
Keywords: H56/CAF01 vaccine; SPECT/CT imaging; T cells; drug delivery; dual-isotope 111In/67Ga; mucosal immunity; nanomedicine; pulmonary immunization.
Figures
Similar articles
-
Intrapulmonary (i.pulmon.) Pull Immunization With the Tuberculosis Subunit Vaccine Candidate H56/CAF01 After Intramuscular (i.m.) Priming Elicits a Distinct Innate Myeloid Response and Activation of Antigen-Presenting Cells Than i.m. or i.pulmon. Prime Immunization Alone.Front Immunol. 2020 May 7;11:803. doi: 10.3389/fimmu.2020.00803. eCollection 2020. Front Immunol. 2020. PMID: 32457748 Free PMC article.
-
Heterologous prime-boost immunization combining parenteral and mucosal routes with different adjuvants mounts long-lived CD4+ T cell responses in lungs.Front Immunol. 2025 Jul 1;16:1599713. doi: 10.3389/fimmu.2025.1599713. eCollection 2025. Front Immunol. 2025. PMID: 40666512 Free PMC article.
-
Parenteral Vaccination With a Tuberculosis Subunit Vaccine in Presence of Retinoic Acid Provides Early but Transient Protection to M. Tuberculosis Infection.Front Immunol. 2019 May 3;10:934. doi: 10.3389/fimmu.2019.00934. eCollection 2019. Front Immunol. 2019. PMID: 31130946 Free PMC article.
-
Immunization strategies against pulmonary tuberculosis: considerations of T cell geography.Adv Exp Med Biol. 2013;783:267-78. doi: 10.1007/978-1-4614-6111-1_14. Adv Exp Med Biol. 2013. PMID: 23468114 Review.
-
Mucosal immunity and novel tuberculosis vaccine strategies: route of immunisation-determined T-cell homing to restricted lung mucosal compartments.Eur Respir Rev. 2015 Jun;24(136):356-60. doi: 10.1183/16000617.00002515. Eur Respir Rev. 2015. PMID: 26028646 Free PMC article. Review.
Cited by
-
Nanocarriers-Assisted Needle-Free Vaccine Delivery Through Oral and Intranasal Transmucosal Routes: A Novel Therapeutic Conduit.Front Pharmacol. 2022 Jan 11;12:757761. doi: 10.3389/fphar.2021.757761. eCollection 2021. Front Pharmacol. 2022. PMID: 35087403 Free PMC article. Review.
-
Repurposing mucosal delivery devices for live attenuated tuberculosis vaccines.Front Immunol. 2023 Mar 30;14:1159084. doi: 10.3389/fimmu.2023.1159084. eCollection 2023. Front Immunol. 2023. PMID: 37063870 Free PMC article.
-
Molecular Imaging of Pulmonary Inflammation and Infection.Int J Mol Sci. 2020 Jan 30;21(3):894. doi: 10.3390/ijms21030894. Int J Mol Sci. 2020. PMID: 32019142 Free PMC article. Review.
-
Carbohydrate Immune Adjuvants in Subunit Vaccines.Pharmaceutics. 2020 Oct 14;12(10):965. doi: 10.3390/pharmaceutics12100965. Pharmaceutics. 2020. PMID: 33066594 Free PMC article. Review.
-
Towards Precision Vaccines: Lessons From the Second International Precision Vaccines Conference.Front Immunol. 2020 Oct 15;11:590373. doi: 10.3389/fimmu.2020.590373. eCollection 2020. Front Immunol. 2020. PMID: 33178222 Free PMC article.
References
-
- WHO Global Tuberculosis Report 2017. Geneva: World Health Organization; (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

