Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-mimicking In Vitro Models in Anti-Cancer Drug Development
- PMID: 30555545
- PMCID: PMC6276092
- DOI: 10.7150/thno.29098
Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-mimicking In Vitro Models in Anti-Cancer Drug Development
Abstract
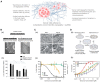
For decades, scientists have been using two-dimensional cell culture platforms for high-throughput drug screening of anticancer drugs. Growing evidence indicates that the results of anti-cancer drug screening vary with the cell culture microenvironment, and this variation has been proposed as a reason for the high failure rate of clinical trials. Since the culture condition-dependent drug sensitivity of anti-cancer drugs may negatively impact the identification of clinically effective drug candidates, more reliable in vitro cancer platforms are urgently needed. In this review article, we provide an overview of how cell culture conditions can alter drug efficacy and highlight the importance of developing more reliable cancer drug testing platforms for use in the drug discovery process. The environmental factors that can alter drug delivery and efficacy are reviewed. Based on these observations of chemoresistant tumor physiology, we summarize the recent advances in the fabrication of in vitro cancer models and the model-dependent cytotoxicity of anti-cancer drugs, with a particular focus on engineered environmental factors in these platforms. It is believed that more physiologically relevant cancer models can revolutionize the drug discovery process.
Keywords: biomimetic; cancer cell; chemoresistance; efficacy; tumor microenvironment.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures




References
-
- Hanahan D. Rethinking the war on cancer. Lancet. 2014;383:558–63. - PubMed
-
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Bio. 2009;10:445–57. - PubMed
-
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

