Novel spheroid reservoir bioartificial liver improves survival of nonhuman primates in a toxin-induced model of acute liver failure
- PMID: 30555564
- PMCID: PMC6276288
- DOI: 10.7150/thno.26540
Novel spheroid reservoir bioartificial liver improves survival of nonhuman primates in a toxin-induced model of acute liver failure
Abstract
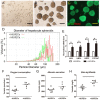
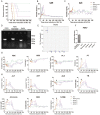
This study aims to evaluate the effectiveness and safety of the spheroid reservoir bioartificial liver (SRBAL) with porcine hepatocyte organoids in a preclinical nonhuman primate model of acute liver failure (ALF). Methods: Thirty healthy rhesus monkeys were infused with α-amanitin and lipopolysaccharide and randomized into five groups (ALF alone control group; sham no-cell SRBAL treatment group; groups A, B and C with SRBAL treatment started at 12 h, 24 h and 36 h after induction of ALF, respectively). Animals were continuously treated with the SRBAL device for 6 h and followed for up to 336 h. Results: Survival of ALF monkeys improved with hepatocyte SRBAL treatment compared to control groups. Blood ammonia and total bilirubin were lower, and albumin levels were higher in all hepatocyte SRBAL treatment groups. No evidence of porcine endogenous retrovirus was identified in monkey liver or blood after SRBAL treatment. Titers of monkey antibody (IgG, IgM) did not rise after SRBAL treatment. In survival cases, the proportion of necrotic and apoptotic hepatocytes was lower in SRBAL-treated groups, with earlier liver regeneration leading to recovery. Cytokines TNF-α, IL-6, IL-12, IL-1β, IL-8, IFN-γ and IL-2 were ameliorated by the SRBAL treatment, while levels of M-CSF; HGF, EGF and VEGF; IL-1RA and MIF rose on priming, proliferation and the late phase of liver regeneration. Conclusions: The benefit of SRBAL therapy included preventive effects and therapeutic effects. SRBAL improved survival rate and prolonged median survival time in a nonhuman primate model of drug-induced ALF, and these benefits declined with a delay in the initiation of therapy. Improved survival and recovery of ALF monkeys was associated with a reduction in blood ammonia levels, inhibition of the pro-inflammatory response of ALF, and provided a microenvironment more suitable for regeneration of the injured liver.
Keywords: Macaca mulatta; acute liver failure; bioartificial liver; hepatocyte; organoid.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2010;369:2525–34. - PubMed
-
- Davies NA, Banares R. A new horizon for liver support in acute liver failure. J Hepatol. 2015;63:303–5. - PubMed
-
- Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289:217. - PubMed
-
- Banares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M. et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153–62. - PubMed
-
- Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B. et al. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med. 2013;159:522–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

