Skin as an immune organ and clinical applications of skin-based immunotherapy
- PMID: 30555619
- PMCID: PMC6284278
- DOI: 10.1186/s40413-018-0215-2
Skin as an immune organ and clinical applications of skin-based immunotherapy
Abstract
Background: The prevalence of food allergy is increasing, and allergen avoidance continues to be the main standard of care. There is a critical need for safe and effective forms of immunotherapy for patients with food allergy as well as other allergic diseases.
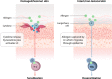
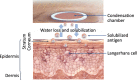
Findings: The skin is a multifunctional organ with unique immunologic properties, making it a favorable administration route for allergen-specific immunotherapy. Epicutaneous immunotherapy (EPIT) takes advantage of the skin's immune properties to modulate allergic responses and is thus one of the allergen-specific immunotherapy approaches currently being investigated for food allergy. Advances made in the understanding of how epicutaneously applied proteins interact with the immune system and in the technology for facilitating such interactions offer many opportunities for clinical application. Research has shown that allergen delivered to intact skin via EPIT is taken up in the superficial layers of the skin by Langerhans cells, avoiding passive movement of allergen through the dermis and limiting systemic circulation. EPIT brings about allergen desensitization by activating a population of regulatory T cells (Tregs) with unique properties and the potential for inducing a sustained effect as well as the possibility (seen in animal models) for protection against further sensitizations. Several clinical trials investigating the therapeutic efficacy of EPIT for treatment of peanut allergy have been completed, as well as a Phase 2 trial for treatment of milk allergy.
Conclusions: Taken together, the reviewed literature supports the concept that EPIT activates the natural desensitization pathway of the skin, offering a progressive, possibly sustained response. EPIT offers a potential alternative for allergen immunotherapy that is less invasive and carries a lower risk for systemic reactions than oral immunotherapy.
Keywords: Allergen immunotherapy; Epicutaneous immunotherapy; Food allergy; Skin.
Conflict of interest statement
Dr. Bird is the director of the Food Allergy Center at Children’s Medical Center in Dallas and is Associate Professor of Pediatrics and Internal Medicine in the Division of Allergy and Immunology at UT Southwestern Medical Center. Dr. Bird’s research interests are in understanding and treating life-threatening reactions to foods, and he is currently working with colleagues in several ongoing clinical trials using food proteins for immunotherapy.Not applicable; the work published here is not a human trial.Not applicable; no human data are involved.Dr. Bird received support for travel to meetings for the study or other purposes; his institution received research support during the conduct of the study from DBV Technologies; he received support as a board member at Food Allergy Research and Education (FARE), consultant fees from Aimmune Therapeutics and Wedbush Consulting, and speaking fees from Aimmune Therapeutics and DBV Technologies; and his institution received research support from Aimmune Therapeutics, DBV Technologies, Foundation of the American College of Allergy, Asthma, and Immunology, and Nestle Health Sciences unrelated to the study. Dr. Sanchez-Borges reports no competing interests. Dr. Ansotegui reports personal fees from Roxall, personal fees from Sanofi, personal fees from UCB, personal fees from Faes Farma, personal fees from Bayer, personal fees from Astra Zeneca, outside the submitted work. Dr. Ebisawa reports personal fees from DBV Technologies outside the submitted work. Dr. Ortega Martell reports personal fees from Autonomous University of Hidalgo State, Mexico, personal fees from UCB Pharma, personal fees from Sanofi Aventis, personal fees from Vifor Pharma, outside the submitted work.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Pawankar R, Canonica GW, Holgate ST, Lockey RF, World Allergy Organization. World Allergy Organization (WAO) White Book on Allergy: WAO; 2011.
-
- Vallery-Radot PHJ. Asthme d’origine équine. Essai de désensibilisation par des cutiréactions répétées. Bull Soc Méd Hôp Paris. 1921;45:1251–1260.
Publication types
LinkOut - more resources
Full Text Sources