Exosomes in cancer therapy: a novel experimental strategy
- PMID: 30555736
- PMCID: PMC6291654
Exosomes in cancer therapy: a novel experimental strategy
Abstract
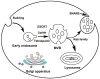
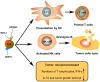
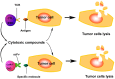
Exosomes are small membrane vesicles of endocytic origin secreted by most cell types. They play important roles in intercellular communications and many physiological processes. DCs-derived exosomes can prime naïve T cells and activate NK cells to shrink the tumor. Tumor-derived exosomes carry a variety of tumor antigens that trigger the robust tumor antigen-specific immune response. Tumor-derived exosomes also contain metastasis or invasive-related molecules, which maybe potential targets for cancer immunotherapy. Effector T cells-derived exosomes possess cytotoxic activity of their original cells, thus cause tumor cells lysis. In this review, we summarized the recent advances on the biogenesis and composition of exosomes, the functions of anti-tumor immune response, and the promising applications on cancer immunotherapy of exosomes from different origins. Exosomes schlep efficient targets homing to tumor sites and tend to be a promising new tool of immunotherapy to fight cancer in a cell-free system.
Keywords: CAR-T cells; CTLs; DCs; Exosomes; cancer immunotherapy.
Conflict of interest statement
None.
Figures

References
-
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. - PubMed
-
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. - PubMed
-
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
