Keratinolytic activity of Bacillus subtilis LFB-FIOCRUZ 1266 enhanced by whole-cell mutagenesis
- PMID: 30555768
- PMCID: PMC6288049
- DOI: 10.1007/s13205-018-1527-1
Keratinolytic activity of Bacillus subtilis LFB-FIOCRUZ 1266 enhanced by whole-cell mutagenesis
Abstract
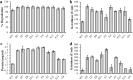
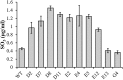
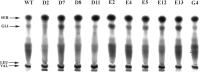
Discarded feathers represent an important residue from the poultry industry and are a rich source of keratin. Bacillus subtilis LFB-FIOCRUZ 1266, previously isolated from industrial poultry wastes, was used in this work and, through random mutation using ethyl methanesulfonate, ten strains were selected based on the size of their degradation halos. The feather degradation was increased to 115% and all selected mutants showed 1.4- to 2.4-fold increase in keratinolytic activity compared to their wild-type counterparts. The protein concentrations in the culture supernatants increased approximately 2.5 times, as a result of feather degradation. The mutants produced more sulfide than the wild-type bacteria that produced 0.45 µg/ml, while mutant D8 produced 1.45 µg/ml. The best pH for enzyme production and feather degradation was pH 8. Zymography showed differences in the intensity and molecular mass of some bands. The peptidase activity of the enzyme blend was predominantly inhibited by PMSF and EDTA, suggesting the presence of serine peptidases. HPTLC analysis evidenced few differences in band intensities of the amino acid profiles produced by the mutant peptidase activities. The mutants showed an increase in keratinolytic and peptidase activities, demonstrating their biotechnological potential to recycle feather and help to reduce the environmental impact.
Keywords: Bacillus; Biodegradation; Keratin; Mutagenesis; Peptidase.
Conflict of interest statement
Compliance with ethical standardsOn behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures


Similar articles
-
Keratinase Production by Three Bacillus spp. Using Feather Meal and Whole Feather as Substrate in a Submerged Fermentation.Enzyme Res. 2011;2011:523780. doi: 10.4061/2011/523780. Epub 2011 Aug 1. Enzyme Res. 2011. PMID: 21822479 Free PMC article.
-
Keratinases and sulfide from Bacillus subtilis SLC to recycle feather waste.World J Microbiol Biotechnol. 2012 Mar;28(3):1259-69. doi: 10.1007/s11274-011-0930-0. Epub 2011 Nov 9. World J Microbiol Biotechnol. 2012. PMID: 22805846
-
Biodegradation of feather waste by extracellular keratinases and gelatinases from Bacillus spp.World J Microbiol Biotechnol. 2011 Jun;27(6):1355-65. doi: 10.1007/s11274-010-0586-1. Epub 2010 Oct 13. World J Microbiol Biotechnol. 2011. PMID: 25187135
-
Keratinolytic activity of Bacillus megaterium F7-1, a feather-degrading mesophilic bacterium.Microbiol Res. 2009;164(4):478-85. doi: 10.1016/j.micres.2007.02.004. Epub 2007 Apr 24. Microbiol Res. 2009. PMID: 17459685
-
Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production.J Basic Microbiol. 2019 Jan;59(1):4-13. doi: 10.1002/jobm.201800434. Epub 2018 Oct 24. J Basic Microbiol. 2019. PMID: 30353928 Review.
Cited by
-
Production of surfactant-stable keratinase from Bacillus cereus YQ15 and its application as detergent additive.BMC Biotechnol. 2022 Sep 8;22(1):26. doi: 10.1186/s12896-022-00757-3. BMC Biotechnol. 2022. PMID: 36076195 Free PMC article.
-
Structure, Application, and Biochemistry of Microbial Keratinases.Front Microbiol. 2021 Jun 23;12:674345. doi: 10.3389/fmicb.2021.674345. eCollection 2021. Front Microbiol. 2021. PMID: 34248885 Free PMC article. Review.
-
Perspectives on Converting Keratin-Containing Wastes Into Biofertilizers for Sustainable Agriculture.Front Microbiol. 2022 Jun 20;13:918262. doi: 10.3389/fmicb.2022.918262. eCollection 2022. Front Microbiol. 2022. PMID: 35794912 Free PMC article. Review.
-
Directed evolution driving the generation of an efficient keratinase variant to facilitate the feather degradation.Bioresour Bioprocess. 2022 Apr 4;9(1):38. doi: 10.1186/s40643-022-00524-4. Bioresour Bioprocess. 2022. PMID: 38647843 Free PMC article.
-
Degradation of tannery hide raw trimming hairs using keratinolytic bacteria isolated from tannery effluent-contaminated soil.Arch Microbiol. 2023 May 13;205(6):235. doi: 10.1007/s00203-023-03571-3. Arch Microbiol. 2023. PMID: 37179267
References
-
- Arikawa Y, Ozawa T, Iwasaki I. An improved photometric method for the determination of sulfite with pararosaniline and formaldehyde. Bull Chem Soc Jpn. 1971;41:1454–1456. doi: 10.1246/bcsj.41.1454. - DOI
-
- Bernal C, Vidal L, Valdivieso E, Coello N. Keratinolytic activity of Kocuria rosea. World J Microbiol Biotechnol. 2003;19:255–261. doi: 10.1023/A:1023685621215. - DOI
LinkOut - more resources
Full Text Sources

