Latent, Immunosuppressive Nature of Poly(lactic- co-glycolic acid) Microparticles
- PMID: 30555893
- PMCID: PMC6290919
- DOI: 10.1021/acsbiomaterials.7b00831
Latent, Immunosuppressive Nature of Poly(lactic- co-glycolic acid) Microparticles
Erratum in
-
Correction to Latent, Immunosuppressive Nature of Poly(lactic-co-glycolic acid) Microparticles.ACS Biomater Sci Eng. 2018 Jun 11;4(6):2224-2225. doi: 10.1021/acsbiomaterials.8b00540. Epub 2018 May 15. ACS Biomater Sci Eng. 2018. PMID: 33435044 No abstract available.
Abstract
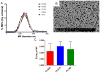
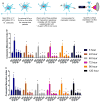
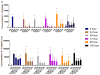
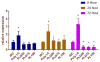
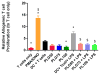
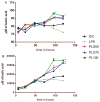
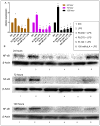
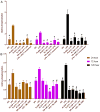
Use of biomaterials to spatiotemporally control the activation of immune cells is at the forefront of biomedical engineering research. As more biomaterial strategies are employed for immunomodulation, understanding the immunogenicity of biodegradable materials and their byproducts is paramount in tailoring systems for immune activation or suppression. Poly(D,L-lactic-co-glycolic acid) (PLGA), one of the most commonly studied polymers in tissue engineering and drug delivery, has been previously described on one hand as an immune adjuvant, and on the other as a nonactivating material. In this study, the effect of PLGA microparticles (MPs) on the maturation status of murine bone marrow-derived dendritic cells (DCs), the primary initiators of adaptive immunity, was investigated to decipher the immunomodulatory properties of this biomaterial. Treatment of bone marrow-derived DCs from C57BL/6 mice with PLGA MPs led to a time dependent decrease in the maturation level of these cells, as quantified by decreased expression of the positive stimulatory molecules MHCII, CD80, and CD86 as well as the ability to resist maturation following challenge with lipopolysaccharide (LPS). Moreover, this immunosuppression was dependent on the molecular weight of the PLGA used to fabricate the MPs, as higher molecular weight polymers required longer incubation to produce comparable dampening of maturation molecules. These phenomena were correlated to an increase in lactic acid both intracellularly and extracellularly during DC/PLGA MP coculture, which is postulated to be the primary agent behind the observed immune inhibition. This hypothesis is supported by our results demonstrating that resistance to LPS stimulation may be due to the ability of PLGA MP-derived lactic acid to inhibit the phosphorylation of TAK1 and therefore prevent NF-κB activation. This work is significant as it begins to elucidate how PLGA, a prominent biomaterial with broad applications ranging from tissue engineering to pharmaceutics, could modulate the local immune environment and offers insight on engineering PLGA to exploit its evolving immunogenicity.
Keywords: dendritic cells; immunogenicity; lactic acid; microparticles; poly(lactic-co-glycolic) acid.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures



References
-
- Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–56. - PubMed
-
- Slawek A, Maj T, Chelmonska-Soyta A. CD40, CD80, and CD86 costimulatory molecules are differentially expressed on murine splenic antigen-presenting cells during the pre-implantation period of pregnancy, and they modulate regulatory T cell abundance, peripheral cytokine response, and pregnancy outcome. Am J Reprod Immunol. 2013;70(2):116–26. - PubMed
-
- Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol Rev. 2005;207:191–205. - PubMed
-
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28(9):2760–9. - PubMed
-
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–71. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
