Chemoenzymatic Approach toward the Synthesis of 3- O-(α/β)-Glucosylated 3-Hydroxy-β-lactams
- PMID: 30556000
- PMCID: PMC6289546
- DOI: 10.1021/acsomega.8b01969
Chemoenzymatic Approach toward the Synthesis of 3- O-(α/β)-Glucosylated 3-Hydroxy-β-lactams
Abstract
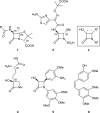
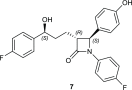
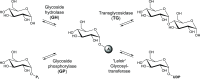
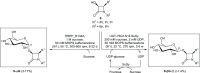
Glycosylation significantly alters the biological and physicochemical properties of small molecules. β-Lactam alcohols comprise eligible substrates for such a transformation based on their distinct relevance in the chemical and medicinal community. In this framework, the unprecedented enzymatic glycosylation of the rigid and highly strained four-membered β-lactam azaheterocycle was studied. For this purpose, cis-3-hydroxy-β-lactams were efficiently prepared in three steps by means of a classical organic synthesis approach, while a biocatalytic step was implemented for the selective formation of the corresponding 3-O-α- and -β-glucosides, hence overcoming the complexities typically encountered in synthetic glycochemistry and contributing to the increasing demand for sustainable processes in the framework of green chemistry. Two carbohydrate-active enzymes were selected based on their broad acceptor specificity and subsequently applied for the α- or β-selective formation of β-lactam-sugar adducts, using sucrose as a glucosyl donor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
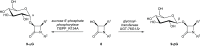

References
-
- Staudinger H. Zur Kenntniss der Ketene. Diphenylketen. Justus Liebigs Ann. Chem. 1907, 356, 51–123. 10.1002/jlac.19073560106. - DOI
-
- Fleming A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236. - PubMed
-
- Bisacchi G. S.; Slusarchyk W. A.; Bolton S. A.; Hartl K. S.; Jacobs G.; Mathur A.; Meng W.; Ogletree M. L.; Pi Z.; Sutton J. C.; Treuner U.; Zahler R.; Zhao G.; Seiler S. M. Synthesis of potent and highly selective nonguanidine azetidinone inhibitors of human tryptase. Bioorg. Med. Chem. Lett. 2004, 14, 2227–2231. 10.1016/j.bmcl.2004.02.011. - DOI - PubMed
LinkOut - more resources
Full Text Sources

