Tissue Engineering for the Temporomandibular Joint
- PMID: 30556348
- PMCID: PMC7075314
- DOI: 10.1002/adhm.201801236
Tissue Engineering for the Temporomandibular Joint
Abstract
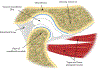
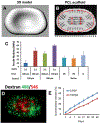
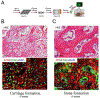
Tissue engineering potentially offers new treatments for disorders of the temporomandibular joint which frequently afflict patients. Damage or disease in this area adversely affects masticatory function and speaking, reducing patients' quality of life. Effective treatment options for patients suffering from severe temporomandibular joint disorders are in high demand because surgical options are restricted to removal of damaged tissue or complete replacement of the joint with prosthetics. Tissue engineering approaches for the temporomandibular joint are a promising alternative to the limited clinical treatment options. However, tissue engineering is still a developing field and only in its formative years for the temporomandibular joint. This review outlines the anatomical and physiological characteristics of the temporomandibular joint, clinical management of temporomandibular joint disorder, and current perspectives in the tissue engineering approach for the temporomandibular joint disorder. The tissue engineering perspectives have been categorized according to the primary structures of the temporomandibular joint: the disc, the mandibular condyle, and the glenoid fossa. In each section, contemporary approaches in cellularization, growth factor selection, and scaffold fabrication strategies are reviewed in detail along with their achievements and challenges.
Keywords: animal studies; cells; growth factors; scaffolds; temporomandibular joint; tissue engineering.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


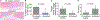




References
-
- Rabie ABM, Dai J, and Xu R, Recombinant AAV-mediated VEGF gene therapy induces mandibular condylar growth. Gene Therapy, 2007. 14: p. 972. - PubMed
-
- Legemate K, et al., Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds. Journal of Dental Research, 2016. 95(7): p. 800–807. - PubMed
-
- Dorland WAN, et al., Dorland’s illustrated medical dictionary. 1957, Philadelphia; London: W. B. Saunders Co.
-
- Helland MM, Anatomy and function of the temporomandibular joint. J Orthop Sports Phys Ther, 1980. 1(3): p. 145–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

