Care prior to and during subsequent pregnancies following stillbirth for improving outcomes
- PMID: 30556599
- PMCID: PMC6516997
- DOI: 10.1002/14651858.CD012203.pub2
Care prior to and during subsequent pregnancies following stillbirth for improving outcomes
Abstract
Background: Stillbirth affects at least 2.6 million families worldwide every year and has enduring consequences for parents and health services. Parents entering a subsequent pregnancy following stillbirth face a risk of stillbirth recurrence, alongside increased risks of other adverse pregnancy outcomes and psychosocial challenges. These parents may benefit from a range of interventions to optimise their short- and longer-term medical health and psychosocial well-being.
Objectives: To assess the effects of different interventions or models of care prior to and during subsequent pregnancies following stillbirth on maternal, fetal, neonatal and family health outcomes, and health service utilisation.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (6 June 2018), along with ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (18 June 2018).
Selection criteria: We included randomised controlled trials (RCTs) and quasi-randomised controlled trials (qRCTs). Trials using a cluster-randomised design were eligible for inclusion, but we found no such reports. We included trials published as abstract only, provided sufficient information was available to allow assessment of trial eligibility and risk of bias. We excluded cross-over trials.
Data collection and analysis: Two review authors independently assessed trials for eligibility and undertook data extraction and 'Risk of bias' assessments. We extracted data from published reports, or sourced data directly from trialists. We checked the data for accuracy and resolved discrepancies by discussion or correspondence with trialists, or both. We conducted an assessment of the quality of the evidence using the GRADE approach.
Main results: We included nine RCTs and one qRCT, and judged them to be at low to moderate risk of bias. Trials were carried out between the years 1964 and 2015 and took place predominantly in high-income countries in Europe. All trials assessed medical interventions; no trials assessed psychosocial interventions or incorporated psychosocial aspects of care. Trials evaluated the use of antiplatelet agents (low-dose aspirin (LDA) or low-molecular-weight heparin (LMWH), or both), third-party leukocyte immunisation, intravenous immunoglobulin, and progestogen. Trial participants were women who were either pregnant or attempting to conceive following a pregnancy loss, fetal death, or adverse outcome in a previous pregnancy.We extracted data for 222 women who had experienced a previous stillbirth of 20 weeks' gestation or more from the broader trial data sets, and included them in this review. Our GRADE assessments of the quality of evidence ranged from very low to low, due largely to serious imprecision in effect estimates as a result of small sample sizes, low numbers of events, and wide confidence intervals (CIs) crossing the line of no effect. Most of the analyses in this review were not sufficiently powered to detect differences in the outcomes assessed. The results presented are therefore largely uncertain.Main comparisonsLMWH versus no treatment/standard care (three RCTs, 123 women, depending on the outcome)It was uncertain whether LMWH reduced the risk of stillbirth (risk ratio (RR) 2.58, 95% CI 0.40 to 16.62; 3 trials; 122 participants; low-quality evidence), adverse perinatal outcome (RR 0.81, 95% CI 0.20 to 3.32; 2 trials; 77 participants; low-quality evidence), adverse maternal psychological effects (RR 1.00, 95% CI 0.07 to 14.90; 1 trial; 40 participants; very low-quality evidence), perinatal mortality (RR 2.58, 95% CI 0.40 to 16.62; 3 trials; 122 participants; low-quality evidence), or any preterm birth (< 37 weeks) (RR 1.01, 0.58 to 1.74; 3 trials; 114 participants; low-quality evidence). No neonatal deaths were reported in the trials assessed and no data were available for maternal-infant attachment. There was no clear evidence of a difference between the groups among the remaining secondary outcomes.LDA versus placebo (one RCT, 24 women)It was uncertain whether LDA reduced the risk of stillbirth (RR 0.85, 95% CI 0.06 to 12.01), neonatal death (RR 0.29, 95% CI 0.01 to 6.38), adverse perinatal outcome (RR 0.28, 95% CI 0.03 to 2.34), perinatal mortality, or any preterm birth (< 37 weeks) (both of the latter RR 0.42, 95% CI 0.04 to 4.06; all very low-quality evidence). No data were available for adverse maternal psychological effects or maternal-infant attachment. LDA appeared to be associated with an increase in birthweight (mean difference (MD) 790.00 g, 95% CI 295.03 to 1284.97 g) when compared to placebo, but this result was very unstable due to the extremely small sample size. Whether LDA has any effect on the remaining secondary outcomes was also uncertain.Other comparisonsLDA appeared to be associated with an increase in birthweight when compared to LDA + LMWH (MD -650.00 g, 95% CI -1210.33 to -89.67 g; 1 trial; 29 infants), as did third-party leukocyte immunisation when compared to placebo (MD 1195.00 g, 95% CI 273.35 to 2116.65 g; 1 trial, 4 infants), but these results were again very unstable due to extremely small sample sizes. The effects of the interventions on the remaining outcomes were also uncertain.
Authors' conclusions: There is insufficient evidence in this review to inform clinical practice about the effectiveness of interventions to improve care prior to and during subsequent pregnancies following a stillbirth. There is a clear and urgent need for well-designed trials addressing this research question. The evaluation of medical interventions such as LDA, in the specific context of stillbirth prevention (and recurrent stillbirth prevention), is warranted. However, appropriate methodologies to evaluate such therapies need to be determined, particularly where clinical equipoise may be lacking. Careful trial design and multicentre collaboration is necessary to carry out trials that would be sufficiently large to detect differences in statistically rare outcomes such as stillbirth and neonatal death. The evaluation of psychosocial interventions addressing maternal-fetal attachment and parental anxiety and depression is also an urgent priority. In a randomised-trial context, such trials may allocate parents to different forms of support, to determine which have the greatest benefit with the least financial cost. Importantly, consistency in nomenclature and in data collection across all future trials (randomised and non-randomised) may be facilitated by a core outcomes data set for stillbirth research. All future trials should assess short- and longer-term psychosocial outcomes for parents and families, alongside economic costs of interventions.
Conflict of interest statement
Aleena M Wojcieszek: none known.
Emily Shepherd: none known.
Philippa Middleton: none known.
Zohra S Lassi: none known.
Trish Wilson: none known.
Margaret M Murphy: none known.
Alexander EP Heazell: Alexander EP Heazell's salary is funded by his National Institute of Health Research (NIHR) Clinician Scientist Award (CS‐2013‐13‐009) although this review is not directly funded by this award. He also receives salary support from Tommy's Charity as Director of the Tommy's Stillbirth Research Centre, University of Manchester. This review is part of this programme of work into improving care in pregnancies after stillbirth. Alexander E P Heazell is the Clinical Lead for a specialist antenatal service for women who have experienced a stillbirth in previous pregnancy.
David A Ellwood: David Ellwood has received sitting fees from the Australian Medical Council but this work is not related to this Cochrane Review. He has received payment for providing expert witness reviews for medico‐legal cases – these cases are in no way related to the topic under review. I am the co‐Director of an NHMRC Centre for Research Excellence ‐ the centre is related to stillbirth and will cover all aspects of research on this topic.
Robert M Silver: Robert M Silver has been awarded NIH grants unrelated to this work. He is a member of the International stillbirth Alliance Scientific Research Committee. He has carried out paid consultancy for Gestavision (a company developing a diagnostic for pre‐eclampsia) and has received payment for grand rounds at several universities.
Vicki Flenady: none known
Figures

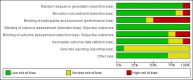
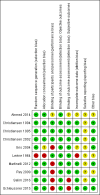









































































































































































































Update of
- doi: 10.1002/14651858.CD012203
References
References to studies included in this review
Ahmed 2014 {published and unpublished data}
-
- Ahmed F, Faryad N, Naeem N. Use of aspirin alone or heparin and aspirin in idiopathic recurrent miscarriages: empirical or evidence based management. Sri Lanka Journal of Obstetrics & Gynaecology 2014;36(Suppl 1):29.
Christiansen 1994 {published and unpublished data}
-
- Christiansen OB, Mathiesen O, Husth M, Lauritsen JG, Grunnet N. Placebo‐controlled trial of active immunization with third party leukocytes in recurrent miscarriage. Acta Obstetricia et Gynecologica Scandinavica 1994;73(3):261‐8. - PubMed
Christiansen 1995 {published and unpublished data}
-
- Christiansen OB, Mathiesen O, Husth M, Rasmussen KL, Ingerslev HJ, Lauritsen JG, et al. Placebo‐controlled trial of treatment of unexplained secondary recurrent spontaneous abortions and recurrent late spontaneous abortions with IV immunoglobulin. Human Reproduction 1995;10(10):2690‐5. - PubMed
Christiansen 2002 {published and unpublished data}
-
- Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double‐blind, placebo‐controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Human Reproduction 2002;17(3):809‐16. - PubMed
Gris 2004 {published and unpublished data}
-
- Gris JC, Mercier E, Quéré I, Lavigne‐Lissalde G, Cochery‐Nouvellon E, Hoffet M, et al. Low‐molecular‐weight heparin versus low‐dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004;103:3695‐9. - PubMed
Levine 1964 {published data only}
-
- LeVine L. Habitual abortion: a controlled clinical study of progestational therapy. Western Journal of Surgery 1964;72:30‐6. - PubMed
Martinelli 2012 {published and unpublished data}
Rey 2009 {published and unpublished data}
-
- Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, et al. Dalteparin for the prevention of recurrence of placental‐mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. Journal of Thrombosis and Haemostasis 2009;7(1):58–64. - PubMed
Salim 2016 {published data only}
-
- Salim R, Nachum Z, Gavish I, Romano S, Braverman M, Garmi G. Adjusting enoxaparin dosage according to anti‐FXa levels and pregnancy outcome in thrombophilic women. A randomised controlled trial. Thrombosis and Haemostasis 2016;116(4):687‐95. - PubMed
Schleussner 2015 {published and unpublished data}
-
- Schleussner E, Kamin G, Seliger G, Rogenhofer N, Ebner S, Toth B, et al. Low‐molecular‐weight heparin for women with unexplained recurrent pregnancy loss: a multicenter trial with a minimization randomization scheme. Annals of Internal Medicine 2015;162(9):601‐9. - PubMed
References to studies excluded from this review
Abdelhafez 2014 {published data only}
-
- NCT02303171. Use of warfarin after the first trimester in pregnant women with antiphospholipid syndrome. clinicaltrials.gov/ct2/show/record/NCT02303171 (first received 27 November 2014).
Ahmadi 2017 {published data only}
-
- Ahmadi M. Immunomodulatory effects of IVIg on pregnancy rate of patient with recurrent pregnancy loss. clinicaltrials.gov/ct2/show/NCT03174951 (first received 5 June 2017).
Alalaf 2012 {published data only}
Aoki 1993 {published data only}
-
- Aoki K, Kajiura S, Matsumoto Y, Yagami Y. Clinical evaluation of immunotherapy in early pregnancy with x‐irradiated paternal mononuclear cells for primary recurrent aborters. American Journal of Obstetrics and Gynecology 1993;169(3):649‐53. - PubMed
Baber 1988 {published data only}
-
- Baber R, Kuan R, Porter R, Saunders D. Early pregnancy support in an in‐vitro fertilization program: does human chorionic gonadotropin reduce the miscarriage rate?. Asia‐Oceania Journal of Obstetrics & Gynaecology 1988;14(4):453‐5. - PubMed
Badawy 2008 {published data only}
-
- Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, Abdelall I. Low‐molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. Journal of Obstetrics and Gynaecology 2008;28(3):280‐4. - PubMed
Bao 2017 {published data only}
-
- Bao SH, Zhou Q, Frempong ST, Tu WY, Sheng SL, Liao H. Use of d‐dimer measurement to guide anticoagulant treatment in recurrent pregnancy loss associated with antiphospholipid syndrome. American Journal of Reproductive Immunology 2017;78(6):e12770. - PubMed
Berle 1980 {published data only}
-
- Berle P, Budenz M, Michaelis J. Is hormonal therapy still justified in imminent abortion?. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184(5):353‐8. - PubMed
Blomqvist 2017 {published data only}
-
- Blomqvist L, Hellgren M, Strandell A. Acetylsalicylic acid for prevention of pregnancy loss: a randomized trial. Human Reproduction 2017;32:i11‐2.
Blumenfeld 1992 {published data only}
-
- Blumenfeld Z, Ruach M. Early pregnancy wastage: the role of repetitive human chorionic gonadotropin supplementation during the first 8 weeks of gestation. Fertility and Sterility 1992;58(1):19‐23. - PubMed
Branch 2000 {published data only}
-
- Branch DW, Peaceman AM, Druzin M, Silver RK, El‐Sayed Y, Silver RM, et al. A multicenter, placebo‐controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy; The Pregnancy Loss Study Group. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 1):122‐7. - PubMed
Brenner 2005 {published data only}
-
- Brenner B, Bar J, Ellis M, Yarom I, Yohai D, Samueloff A, for the Live‐Enox Investigators. Effects of enoxaparin on late pregnancy complications and neonatal outcome in women with recurrent pregnancy loss and thrombophilia: results from the live‐enox study. Fertility and Sterility 2005;84(3):770‐3. - PubMed
Carta 2005 {published data only}
-
- Carta G, Iovenitti P, Falciglia K. Recurrent miscarriage associated with antiphospholipid antibodies: prophylactic treatment with low‐dose aspirin and fish oil derivates. Clinical and Experimental Obstetrics & Gynecology 2005;32(1):49‐51. - PubMed
Cauchi 1991 {published data only}
-
- Cauchi MN, Lim D, Young DE, Kloss M, Pepperell RJ. Treatment of recurrent aborters by immunization with paternal cells ‐‐ controlled trial. American Journal of Reproductive Immunology 1991;25(1):16‐7. - PubMed
Chakravarty 2012 {published data only}
-
- Chakravarty BN, Ganesh A, Chowdhuri K, Shyam T, Ghosh S, Chattopadhyay R. Assessment of endometrial vascularity following dydrogesterone and micronized progesterone administration in idiopathic recurrent miscarriage‐a preliminary study. Human Reproduction 2012;27(Suppl 2):P089.
Check 1995 {published data only}
-
- Check JH, Tarquini P, Gandy P, Lauer C. A randomized study comparing the efficacy of reducing the spontaneous abortion rate following lymphocyte immunotherapy and progesterone treatment vs progesterone alone in primary habitual aborters. Gynecologic and Obstetric Investigation 1995;39(4):257‐61. - PubMed
Christiansen 1992 {published data only}
-
- Christiansen OB, Christiansen BS, Husth M, Mathiesen O, Lauritsen J, Grunnet N. Prospective study of anticardiolipin antibodies in immunized and untreated women with recurrent spontaneous abortions. Fertility and Sterility 1992;58(2):328‐34. - PubMed
Christiansen 2015 {published data only}
-
- Christiansen O, Larsen E, Egerup P, Lunoee L, Egestad L, Nielsen H. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, double‐blind, placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122(4):500‐8. - PubMed
Clark 2010 {published data only}
-
- Clark P, Walker ID, Langhorne P, Crichton L, Thomson A, Greaves M, et al. on behalf of the Scottish Pregnancy Intervention Study collaborators. SPIN (Scottish Pregnancy Intervention) study: a multicenter, randomized controlled trial of low‐molecular‐weight heparin and low‐dose aspirin in women with recurrent miscarriage. Blood 2010;115(21):4162‐7. - PubMed
Cohen 1996 {published data only}
-
- Cohen H. Randomized trial of aspirin versus aspirin and heparin in pregnant women with the antiphospholipid syndrome. Annales de Medecine Interne 1996;147(Suppl 1):44. - PubMed
Coomarasamy 2015 {published data only}
-
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. New England Journal of Medicine 2015;373(22):2141‐8. - PubMed
Cote‐Arsenault 2014 {published data only}
-
- Cote‐Arsenault D, Krowchuk H, Schwartz K, McCoy P. Evidence‐based intervention with women pregnant after perinatal loss. MCN: The American Journal of Maternal Child Nursing 2014;39(3):177‐86. - PubMed
Coulam 1995 {published data only}
-
- Coulam CB, Krysa L, Stern JJ, Bustillo M. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. American Journal of Reproductive Immunology 1995;34(6):333‐7. - PubMed
Cowchock 1992 {published data only}
-
- Cowchock FS, Reece EA, Balaban D, Branch DW, Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low‐dose heparin treatment. American Journal of Obstetrics and Gynecology 1992;166(5):1318‐23. - PubMed
Cowchock 1995 {published data only}
-
- Cowchock FS, Smith JB. Fertility among women with recurrent spontaneous abortions ‐ the effect of paternal cell immunization treatment. American Journal of Reproductive Immunology 1995;33(2):176‐81. - PubMed
Cowchock 1997 {published data only}
-
- Cowchock S, Reece EA. Do low‐risk pregnant women with antiphospholipid antibodies need to be treated? Organizing group of the antiphospholipid antibody treatment trial. American Journal of Obstetrics and Gynecology 1997;176(5):1099‐100. - PubMed
Dal Canto 2012 {published data only}
-
- Dal Canto M. Prospective randomized controlled trial to assess the efficacy of embryogen culture medium to improve ongoing pregnancy and implantation rates in ivf treatments of patients with a previous history of pregnancy loss. clinicaltrials.gov/ct2/show/NCT01689428 (first received 21 September 2012).
Dendrinos 2007 {published data only}
-
- Dendrinos S, Kalogirou I, Makrakis E, Theodoridis T, Mahmound EA, Christopoulou‐Cokkinou V, et al. Safety and effectiveness of tinzaparin sodium in the management of recurrent pregnancy loss. Clinical and Experimental Obstetrics & Gynecology 2007;34(3):143‐5. - PubMed
DeVeciana 2001 {published data only}
-
- Veciana M, Trail P, Dattel B, Slotnick RN, Abuhamad A. Dalteparin versus unfractionated heparin for prophylactic anticoagulation during pregnancy. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S182.
Dolitzky 2006 {published data only}
-
- Dolitzky M, Inbal A, Segal Y, Weiss A, Brenner B, Carp H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertility and Sterility 2006;86(2):362‐6. - PubMed
Elmahashi 2014 {published data only}
El‐Zibdeh 2005 {published data only}
-
- El‐Zibdeh, MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry and Molecular Biology 2005;97(5):431‐4. - PubMed
Epperson 2011 {published data only}
-
- Epperson CN. Effectiveness of cognitive processing therapy in pregnant women with a history of pregnancy loss/complication. clinicaltrials.gov/ct2/show/results/NCT01277354 (first received 14 January 2011).
Famina 2015 {published data only}
-
- Famina M. The effect of dydrogesterone on placental angiogenesis and pregnancy outcomes in women with threatened miscarriages. Giornale Italiano di Ostetricia e Ginecologia 2015;37(4):155‐8.
Farquharson 2002 {published data only}
-
- Farquharson RG, Quenby S. Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstetrics and Gynecology 2002;100(3):408‐13. - PubMed
Fawzy 2008 {published data only}
-
- Fawzy M, Shokeir T, El‐Tatongy M, Warda O, El‐Refaiey AA, Mosbah A. Treatment options and pregnancy outcome in women with idiopathic recurrent miscarriage: a randomized placebo‐controlled study. Archives of Gynecology and Obstetrics 2008;278(1):33‐8. - PubMed
Fuchs 1966 {published data only}
-
- Fuchs F, Olsen P. An attempted double‐blind controlled trial of progesterone therapy in habitual abortion. Ugeskrift for Laeger 1966;128:1461‐2. - PubMed
Gao 2015 {published data only}
-
- Gao HY, Tao EX, Wang Y, Yue QA, Ren CE, Yan LF. Immunomodulatory and clinical effects of the "tiaomian iii decoction" in patients with blood blocking antibody deficiency and recurrent spontaneous abortion. Genetics and Molecular Research 2015;14(2):3421‐5. - PubMed
Gatenby 1993 {published data only}
-
- Gatenby PA, Cameron K, Simes J, Adelstein S, Bennett MJ, Jansen RP, et al. Treatment of recurrent spontaneous abortion by immunization with paternal lymphocytes: results of a controlled trial. American Journal of Reproductive Immunology 1993;29(2):88‐94. - PubMed
Gerhard 1987 {published data only}
-
- Gerhard I, Gwinner B, Eggert‐Kruse W, Runnebaum B. Double‐blind controlled trial of progesterone substitution in threatened abortion. Biological Research in Pregnancy and Perinatology 1987;8(1):26‐34. - PubMed
German RSA/IVIG Group 1994 {published data only}
-
- The German RSA/IVIG Group. Intravenous immunoglobulin in the prevention of recurrent miscarriage: The German RSA/IVIG Group. British Journal of Obstetrics and Gynaecology 1994;101(12):1072‐7. - PubMed
Geva 1998 {published data only}
-
- Geva E, Amit A, Lerner‐Geva L, Lessing JB. Prevention of early pregnancy loss in autoantibody seropositive women. Lancet 1998;351(9095):34‐5. - PubMed
Giancotti 2012 {published data only}
-
- Giancotti A, Torre RL, Spagnuolo A, D'Ambrosio V, Cerekja A, Piazze J, et al. Efficacy of three different antithrombotic regimens on pregnancy outcome in pregnant women affected by recurrent pregnancy loss. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(7):1191‐4. - PubMed
Goel 2006 {published data only}
-
- Goel N, Tuli A, Choudhry R. The role of aspirin versus aspirin and heparin in cases of recurrent abortions with raised anticardiolipin antibodies. Medical Science Monitor 2006;12(3):132‐6. - PubMed
Goldzieher 1964 {published data only}
-
- Goldzieher JW. Double‐blind trial of a progestin in habitual abortion. JAMA 1964;188(7):651‐4. - PubMed
Gomaa 2014 {published data only}
-
- Gomaa MF, Elkholy AG, El‐Said MM, Abdel‐Salam NE. Combined oral prednisolone and heparin versus heparin: the effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. A double‐blind placebo randomized controlled trial. Archives of Gynecology and Obstetrics 2014;290(4):757‐62. - PMC - PubMed
Gris 1995 {published data only}
-
- Gris J, Neveu S, Tailland M, Courtieu C, Mares P, Schved J. Use of a low‐molecular weight heparin (enoxaparin) or of a phenformin‐like substance (moroxydine chloride) in primary early recurrent aborters with an impaired fibrinolytic capacity. Thrombosis and Haemostasis 1995;73(3):362‐7. - PubMed
Gris 2010 {published data only}
-
- Gris JC, Chauleur C, Faillie JL, Baer G, Mares P, Fabbro‐Peray P, et al. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae: the pilot randomised controlled NOH‐AP trial. Thrombosis and Haemostasis 2010;104(4):771‐9. - PubMed
Gris 2011 {published data only}
-
- Gris JC, Chauleur C, Molinari N, Mares P, Fabbro‐Peray P, Quere I, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre‐eclampsia: The pilot randomised controlled NOH‐PE trial. Thrombosis and Haemostasis 2011;106(6):1053‐61. - PubMed
Harrison 1992 {published data only}
-
- Harrison, RF. Human chorionic gonadotrophin (hCG) in the management of recurrent abortion; results of a multi‐centre placebo‐controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1992;47(3):175‐9. - PubMed
Ho 1991 {published data only}
-
- Ho HN, Gill TJ, Hsieh HJ, Jiang JJ, Lee TY, Hsieh CY. Immunotherapy for recurrent spontaneous abortions in a Chinese population. American Journal of Reproductive Immunology 1991;25(1):10‐5. - PubMed
Illeni 1994 {published data only}
-
- Illeni MT, Marelli G, Parazzini F, Acaia B, Bocciolone L. Bontempelli M, et al. Immunotherapy and recurrent abortion: a randomized clinical trial. Human Reproduction 1994;9(7):1247‐9. - PubMed
Ismail 2016 {published data only}
-
- Ismail AM, Hamed AH, Saso S, Abu‐Elhasan AM, Abu‐Elghar MM, Abdelmeged AN. Randomized controlled study of pre‐conception thromboprophylaxis among patients with recurrent spontaneous abortion related to antiphospholipid syndrome. International Journal of Gynecology and Obstetrics 2016;132(2):219‐23. - PubMed
Ismail 2018 {published data only}
-
- Ismail AM, Abbas AM, Ali MK, Amin AF. Peri‐conceptional progesterone treatment in women with unexplained recurrent miscarriage: a randomized double‐blind placebo‐controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2018;31(3):388‐94. - PubMed
Jablonowska 1999 {published data only}
-
- Jablonowska B, Selbing A, Palfi M, Ernerudh J, Kjellberg S, Lindton B. Prevention of recurrent spontaneous abortion by intravenous immunoglobulin: a double‐blind placebo‐controlled study. Human Reproduction 1999;14(3):838‐41. - PubMed
Johnson 1975 {published data only}
-
- Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha‐hydroxyprogesterone caproate in the prevention of premature labor. New England Journal of Medicine 1975;293(14):675‐80. - PubMed
Johnson 1991 {published data only}
-
- Johnson PM, Ramsden GH, Chia KV, Hart CA, Farquharson RG, Francis WJA. A combined randomised double‐blind and open study of trophoblast membrane infusion (TMI) in unexplained recurrent miscarriage. Cellular and Molecular Biology of the Materno‐Fetal Relationship. Vol. 212, John Libbey Eurotext Ltd, 1991:277‐84.
Kaaja 1993 {published data only}
-
- Kaaja R, Julkunen H, Viinikka L, Ylikorkala O. Production of prostacylin and thromboxane in lupus pregnancies: effect of small dose of aspirin. Obstetrics and Gynecology 1993;81(3):327‐31. - PubMed
Kaandorp 2010 {published data only}
-
- Kaandorp SP, Goddijn M, Post JA, Hutten BA, Verhoeve HR, Hamulyák K, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. New England Journal of Medicine 2010;362(17):1586‐96. - PubMed
Kayatas 2013 {published data only}
-
- Kayatas S, Yuksel H, Ertekin A, Cam C. Comparison of tinzaparin with enoxaparin in unexplained recurrent miscarriage. Fertility and Sterility 2013;100(3 Suppl 1):S319.
Khan 2017 {published data only}
-
- Khan ES, Basharat A, Jamil M, Ayub S, Khan MA. Preventive role of low‐molecular‐weight heparin in unexplained recurrent pregnancy loss. South African Journal of Obstetrics and Gynaecology 2017;23(1):17‐9.
Kilpatrick 1993 {published data only}
-
- Kilpatrick DC, Kitchin AJ, Liston WA. Humoral immune response to lymphocyte antigens in early pregnancy and after leucocyte immunotherapy. Journal of Obstetrics and Gynaecology 1993;13:77‐81.
Kim 1997 {published data only}
-
- Kim CH, Chae HD, Koo JN, Kim NY, Kang BM, Chi HS. Comparison of pregnancy outcome between low dose aspirin alone and aspirin plus prednisolone treatment in recurrent spontaneous abortion associated with antiphospholipid antibodies. Korean Journal of Obstetrics and Gynecology 1997;40(7):1404‐11.
Kim 2012 {published data only}
-
- Kim CH, Lee KH, Kim SH, Chae HD, Kang BM, Jung KS. Effect of etanercept treatment in women with unexplained primary recurrent pregnancy loss. Human Reproduction 2012;27(Suppl 2):P098.
Klopper 1965 {published data only}
-
- Klopper AI, MacNaughton MC. Hormones in recurrent abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1022‐8.
Kumar 2014 {published data only}
-
- Kumar A, Begum N, Prasad S, Aggarwal S, Sharma S. Oral dydrogesterone treatment during early pregnancy to prevent recurrent pregnancy loss and its role in modulation of cytokine production: a double‐blind, randomized, parallel, placebo‐controlled trial. Fertility and Sterility 2014;102(5):1357‐63.e3. - PubMed
Kutteh 1996 {published data only}
-
- Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody‐associated recurrent pregnancy loss with lower dose heparin and aspirin. American Journal of Reproductive Immunology 1996;35(4):402‐7. - PubMed
Kwon 2012 {published data only}
-
- Kwon SK, Kim CH, Ahn JW, Lee KH, Chae HD, Kang BM. Effect of intravenous immunoglobulin on pregnancy outcome following IVF/ICSI in infertile patients with endometriosis. Fertility and Sterility 2012;98 Suppl 1(3):S263 Abstract no:O‐511.
Laskin 1997 {published data only}
-
- Laskin CA, Bombardier C, Hannah ME, Mandel FP, Ritchie JW, Farewell V, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. New England Journal of Medicine 1997;337(3):148‐53. - PubMed
Laskin 2009 {published data only}
-
- Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker G, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. Journal of Rheumatology 2009;36(2):279‐87. - PubMed
Lazzarin 2009 {published data only}
-
- Lazzarin N, Vaquero E, Exacoustos C, Bertonotti E, Romanini ME, Arduini D. Low‐dose aspirin and omega‐3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertility and Sterility 2009;92(1):296‐300. - PubMed
Li 1998 {published data only}
-
- Li D, Li C, Zhu Y. Comparative study of the third party and paternal leukocyte immunization in recurrent spontaneous abortion of lowered maternal‐fetal immuno‐recognition. Zhonghua Fu Chan Ke Za Zhi [Chinese Journal of Obstetrics & Gynecology] 1998;33(10):597‐600. - PubMed
MacDonald 1972 {published data only}
-
- MacDonald RR, Goulden R, Oakey RE. Cervical mucus, vaginal cytology and steroid excretion in recurrent abortion. Obstetrics and Gynecology 1972;40(3):394‐402. - PubMed
Maged 2016 {published data only}
-
- Maged AM, Abdelhafiz A, Mostafa WA, El‐Nassery N, Fouad M, Salah E, et al. The role of prophylactic use of low dose aspirin and calheparin in patients with unexplained recurrent abortion. Gynecological Endocrinology 2016;32(12):970‐2. - PubMed
Mahmoud 2004 {published data only}
-
- Mahmoud F, Diejomaoh M, Omu A, Abul H, Haines D. Effect of IgG therapy on lymphocyte subpopulations in the peripheral blood of Kuwaiti women experiencing recurrent pregnancy loss. Gynecologic and Obstetric Investigation 2004;58(2):77‐83. - PubMed
Malathi 2011 {published data only}
-
- Malathi V. Treatment outcome in women suffering from recurrent miscarriages and antiphospholipid syndrome. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:184.
Malinowski 2003 {published data only}
-
- Malinowski A, Dynski MA, Maciolek‐Blewniewska G, Glowacka E, Pawlowski T, Babula G. Treatment outcome in women suffering from recurrent miscarriages and antiphospholipid syndrome. Ginekologia Polska 2003;74(10):1213‐22. - PubMed
Mankuta 1999 {published data only}
-
- Mankuta D, Spitzer KA, Seaward G, Farine D, Ryan G, Clark‐Soloninka CA, et al. Prednisone does not affect the biophysical score in pregnant women with autoantibodies. American Journal of Obstetrics and Gynecology 1999;180:S163.
Meng 2016 {published data only}
-
- Meng L, Lin J, Chen L, Wang Z, Liu M, Liu Y, et al. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Archives of Gynecology and Obstetrics 2016;294(1):29‐39. - PubMed
Mohamed 2014 {published data only}
-
- Mohamed KA, Saad AS. Enoxaparin and aspirin therapy for recurrent pregnancy loss due to anti‐phospholipid syndrome (APS). Middle East Fertility Society Journal 2014;19(3):176‐82.
Moller 1965 {published data only}
-
- Moller KJ, Fuchs F. Double blind controlled trial of 6‐methyl‐17‐acetoxyprogesterone in threatened abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1042‐4.
Mowbray 1985 {published data only}
-
- Mowbray JF, Gibbings C, Liddell H, Reginald PW, Underwood JL, Beard RW. Controlled trial of treatment of recurrent spontaneous abortion by immunisation with paternal cells. Lancet 1985;1(8435):941‐3. - PubMed
Nagpal 2001 {published data only}
-
- Nagpal M, Malhotra R. Should human chorionic gonadotropin supplementation be used as a routine prophylaxis in high risk pregnancies?. Journal of Obstetrics and Gynecology of India 2001;51(4):65‐7.
Navidian 2018 {published data only}
-
- Navidian A, Saravani Z. Impact of cognitive behavioral‐based counseling on grief symptoms severity in mothers after stillbirth. Iranian Journal of Psychiatry and Behavioral Sciences 2018;12(1):e9275.
Nazari 2015 {published data only}
Noble 2005 {published data only}
-
- Noble LS, Kutteh WH, Lashey N, Franklin RD, Herrada J. Antiphospholipid antibodies associated with recurrent pregnancy loss: prospective, multicenter, controlled pilot study comparing treatment with low‐molecular‐weight heparin versus unfractionated heparin. Fertility and Sterility 2005;83(3):684‐90. - PubMed
Norman 2006 {published data only}
-
- Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double‐blind, placebo‐controlled study and meta‐analysis. Lancet 2009;373(9680):2034‐40. - PubMed
Norman 2016 {published data only}
Ober 1999 {published data only}
-
- Ober C, Karrison T, Odem RR, Barnes RB, Branch DW, Stephenson MD, et al. Mononuclear‐cell immunisation in prevention of recurrent miscarriages: a randomised trial. Lancet 1999;354(9176):365‐9. - PubMed
Pandey 2004 {published data only}
-
- Pandey MK, Agrawal S. Induction of MLR‐Bf and protection of fetal loss: a current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. International Immunopharmacology 2004;4(2):289‐98. - PubMed
Pasquier 2015 {published data only}
Pattison 2000 {published data only}
-
- Pattison NS, Chamley LW, Birdsall M, Zanderigo AM, Liddell HS, McDougall J. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2000;183(4):1008‐12. - PubMed
Perino 1997 {published data only}
-
- Perino A, Vassiliadis A, Vucetich A, Colacurci N, Menato G, Cignitti M, et al. Short‐term therapy for recurrent abortion using intravenous immunoglobulins: results of a double‐blind placebo‐controlled Italian study. Human Reproduction 1997;12(11):2388‐92. - PubMed
Prietl 1992 {published data only}
-
- Prietl G, Diedrich K, Ven HH, Luckhaus J, Krebs D. The effect of 17alpha‐hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: a prospective and randomized controlled trial. Human Reproduction 1992;7(1):1‐5. - PubMed
Quenby 1992 {published data only}
-
- Quenby S, Farquharson R, Ramsden G. The obstetric outcome of patients with positive anticardiolipin antibodies: aspirin vs no treatment. 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:443.
Quenby 1994 {published data only}
-
- Quenby S, Farquharson RG. Human chorionic gonadotropin supplementation in recurring pregnancy loss: a controlled trial. Fertility and Sterility 1994;62(4):708‐10. - PubMed
Quenby 2007 {published data only}
-
- Quenby S. A randomised controlled trial of prednisolone for women with recurrent miscarriage and high levels of uterine natural killer cells in the endometrium. isrctn.com/ISRCTN28090716 (first received 5 July 2007).
Qureshi 2005 {published data only}
-
- Qureshi NS, Edi‐Osagie EC, Ogbo V, Ray S, Hopkins R. First trimester threatened miscarriage treatment with human chorionic gonadotrophins: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112(11):1536‐41. - PubMed
Raddatz 2005 {published data only}
-
- Raddatz G. Habitual abortion study: oral dydrogesterone treatment during pregnancy in women with recurrent miscarriage. clinicaltrials.gov/ct2/show/NCT00193674 (first received 19 September 2005).
Rafiee 2015 {published data only}
-
- Rafiee M, Gharagozloo M, Ghahiri A, Mehrabian F, Maracy MR, Kouhpayeh S, et al. Altered th17/treg ratio in recurrent miscarriage after treatment with paternal lymphocytes and vitamin d3: a double‐blind placebo‐controlled study. Iranian Journal of Immunology 2015;12(4):252‐62. - PubMed
Rai 1997 {published data only}
Rai 2005 {published data only}
-
- Rai R. Steroids and antiphospholipid syndrome ‐ related pregnancy loss. clinicaltrials.gov/ct2/show/NCT00180778 (first received 16 September 2005).
Rajan 1993 {published data only}
-
- Rajan L, Oakley A. No pills for heartache: the importance of social support for women who suffer pregnancy loss. Journal of Reproductive and Infant Psychology 1993;11(2):75‐87.
Reijnders 1988 {published data only}
-
- Reijnders FJ, Thomas CM, Doesburg WH, Rolland R, Eskes T. Endocrine effects of 17 alpha‐hydroxyprogesterone caproate during early pregnancy: a double‐blind clinical trial. British Journal of Obstetrics and Gynaecology 1988;95(5):462‐8. - PubMed
Reznikoff‐Etievant 1994 {published data only}
-
- Reznikoff‐Etievant MF. Abstracts of contributors' individual data submitted to the worldwide prospective observation study on immunotherapy for treatment of recurrent spontaneous abortion. American Journal of Reproductive Immunology 1994;32:266‐7.
Rodger 2014 {published data only}
-
- Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, Sermer M, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open‐label randomised trial. Lancet 2014;384(9955):1673‐83. - PubMed
Saad 2014 {published data only}
-
- Saad A, Mohamed K. Pregnancy outcomes in women with recurrent miscarriage treated with low dose aspirin and unfractionated heparin. clinicaltrials.gov/ct2/show/NCT02144064 (first received 21 May 2014).
Salman 2012 {published data only}
-
- Salman SA, Shaaban OM, Zahran KM, Fathalla MM, Anan MA. Low molecular weight heparin (LMWH) for treatment of recurrent miscarriage negatively tested for anti phospholipid antibodies: A randomized controlled trial. Fertility and Sterility 2012;98(3 Suppl 1):S191.
Samantha 2013 {published data only}
-
- Samantha P, Barillari G, Venturelli U, Turello M. Thromboprophylaxis with two different dosages of LMWH (calcic nadroparin) in pregnant women with thrombophilia: a single center experience. Journal of Thrombosis and Haemostasis 2013;11:96‐7.
Scarpellini 2009 {published data only}
-
- Scarpellini F, Sbracia M. Use of granulocyte colony‐stimulating factor for the treatment of unexplained recurrent miscarriage: a randomised controlled trial. Human Reproduction 2009;24(11):2703‐8. - PubMed
Scarpellini 2017 {published data only}
-
- Scarpellini F, Balili A, Sbracia M. G‐CSF treatment in unexplained recurrent pregnancy loss: Results of a controlled trial on 120 women and total data on ten years experience. Journal of Perinatal Medicine 2017;45(Suppl 1):28.
Schisterman 2014 {published data only}
Scott 1996 {published data only}
-
- Scott JR, Branch WD, Dudley D. [personal communication]. Letter to: Cochrane Pregnancy and Childbirth Group 23 August 1996.
Shaaban 2017 {published data only}
-
- Shaaban OM, Abbas AM, Zahran KM, Fathalla MM, Anan MA, Salman SA. Low‐molecular‐weight heparin for the treatment of unexplained recurrent miscarriage with negative antiphospholipid antibodies: a randomized controlled trial. Clinical and Applied Thrombosis/Hemostasis 2017;23(6):567‐72. - PubMed
Sharifi Saki 2015 {published data only}
-
- Sharifi Saki S. Effectiveness of mindfulness‐based cognitive therapy group training in reducing anxiety and meta‐worry of women having repeated spontaneous abortion. en.irct.ir/trial/18498 (first received 17 July 2015).
Shearman 1963 {published data only}
Shefras 1995 {published data only}
-
- Shefras J, Farquharson RG. Heparin therapy, bone density and pregnancy. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:93.
Shu 2002 {published data only}
-
- Shu J, Miao P, Wang RJ. Clinical observation on effect of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody‐positive early recurrent spontaneous abortion. Chinese Journal of Integrated Traditional and Western Medicine 2002;22(6):414‐6. - PubMed
Silver 1993 {published data only}
-
- Silver RK, MacGregor SN, Sholl JS, Hobart JM, Neerhof MG, Ragin A. Comparative trial of prednisone plus aspirin vs aspirin alone in the treatment of anticardiolipin antibody‐positive obstetric patients. American Journal of Obstetrics and Gynecology 1993;169(6):1411‐7. - PubMed
Smitz 1992 {published data only}
-
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem AC. Randomized prospective trial comparing supplementation of the luteal phase and of early pregnancy by natural progesterone given by intramuscular or vaginal administration. Revue Francaise de Gynecologie et d Obstetrique 1992;10(87):507‐16. - PubMed
Sondergaard 1985 {published data only}
-
- Sondergaard F, Ottesen B, Detlefsen GU, Schierup L, Pederson SC, Lebech PE. Progesterone treatment of cases of threatened pre‐term delivery in women with a low level of plasma progesterone. Contraception, Fertilité et Sexualité 1985;13:1227‐31.
Stephenson 2004 {published data only}
-
- Stephenson MD, Ballem PJ, Tsang P, Purkiss S, Ensworth S, Houlihan E, et al. Treatment of antiphospholipid antibody syndrome (APS) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. Journal of Obstetrics and Gynaecology Canada: JOGC 2004;26(8):729‐34. - PubMed
Stephenson 2010 {published data only}
Stray‐Pedersen 1996 {published data only}
-
- Stray‐Pedersen S. [personal communication]. Letter to: Cochrane Pregnancy and Childbirth Group 23 August 1996.
Sun 2010 {published data only}
-
- Sun X‐G, Liu XY, Zhu R, Fan GS, Zhang Y, Chen FL. Effectiveness of intravenous immunoglobulin therapy in treating unexplained recurrent spontaneous abortion and its effect on the level of serum soluble human leucocyte antigen G. Acta Academiae Medicinae Sinicae 2010;32(5):483‐7. - PubMed
Svigos 1982 {published data only}
-
- Svigos J. Preliminary experience with the use of human chorionic gonadotrophin therapy in women with repeated abortion. Clinical Reproduction and Fertility 1982;1(2):131‐5. - PubMed
Swyer 1953 {published data only}
Tang 2013 {published data only}
-
- Tang AW, Alfirevic Z, Turner MA, Drury JA, Small R, Quenby S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Human Reproduction 2013;28(7):1743‐52. - PubMed
Tognoni 1980 {published data only}
-
- Tognoni G, Ferrario L, Inzalaco M, Crosignani PG. Progestagens in threatened abortion. Lancet 1980;2(8206):1242‐3. - PubMed
Triolo 2003 {published data only}
-
- Triolo G, Ferrante A, Ciccia F, Accardo‐Palumbo A, Perino A. Castelli A, et al. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis and Rheumatism 2003;48(3):728‐31. - PubMed
Tulppala 1997 {published data only}
-
- Tulppala M, Marttunen M, Soderstrom‐Anttila V, Ailus K, Palosuo T, Ylikorkala O. Low dose aspirin in the prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Human Reproduction 1997;12(1):191. - PubMed
Turner 1966 {published data only}
-
- Turner SJ, Mizock GB, Feldman GL. Prolonged gynecologic and endocrine manifestations subsequent to administration of medroxyprogesterone acetate during pregnancy. American Journal of Obstetrics and Gynecology 1966;95(2):222‐7. - PubMed
Vahid Dastjerdi 1999 {published data only}
-
- Vahid Dastjerdi M, Moini A, Aleyasin A, Kashaf H, Marsoosi V, Aghahosscini A. The effect of acetyl salicylic acid and prednisolone before and during pregnancy in reducing unexplained recurrent abortions. Fertility and Sterility 1999;72:S203.
Van Hoorn 2016 {published data only}
-
- Hoorn ME, Hague WM, Pampus MG, Bezemer D, Vries JI. Low‐molecular‐weight heparin and aspirin in the prevention of recurrent early‐onset pre‐eclampsia in women with antiphospholipid antibodies: the FRUIT‐RCT. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;197:168‐73. - PubMed
Vaquero 2001 {published data only}
-
- Vaquero E, Lazzarin N, Valensise H, Menghini S, Pierro G, Cesa F, et al. Pregnancy outcome in recurrent spontaneous abortion associated with antiphospholipid antibodies: a comparative study of intravenous immunoglobulin versus prednisone plus low‐dose aspirin. American Journal of Reproductive Immunology 2001;45(3):174‐9. - PubMed
Visser 2011 {published data only}
-
- Visser J, Ulander VM, Helmerhorst FM, Lampinen K, Morin‐Papunen L, Bloemenkamp KW, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia ‐ HABENOX*: a randomised multicentre trial. Thrombosis and Haemostasis 2011;105(2):295‐301. - PubMed
Walch 2005 {published data only}
-
- Walch K, Hefler L, Nagele F. Oral dydrogesterone treatment during the first trimester of pregnancy: the prevention of miscarriage study (PROMIS). A double‐blind, prospectively randomized, placebo‐controlled, parallel group trial. Journal of Maternal‐fetal & Neonatal Medicine 2005;18(4):265‐9. - PubMed
Xiao 2013 {published data only}
Zafardoust 2017 {published data only}
-
- Zafardoust S, Mehdi Akhondi M, Sadeghi Mohammad R, Mohammadzadeh A, Karimi A, Jouhari S, et al. Efficacy of intrauterine injection of granulocyte colony stimulating factor (g‐csf) on treatment of unexplained recurrent miscarriage: a pilot rct study. Journal of Reproduction & Infertility 2017;18(4):379‐85. - PMC - PubMed
Zolghadri 2010 {published data only}
-
- Zolghadri J, Ahmadpour F, Momtahan M, Tavana Z, Foroughinia L. Evaluation of the efficacy of aspirin and low molecular weight heparin in patients with unexplained recurrent spontaneous abortionsIranian. Red Crescent Medical Journal 2010;12(5):548‐52.
References to ongoing studies
Alves 2014 {published data only}
De Jong 2015 {published data only}
El‐refaie 2016 {published data only}
-
- El‐refaie W. Vaginal progesterone versus cervical cerclage for pregnant women with short cervix and history of PTL and/or MTM. clinicaltrials.gov/ct2/show/NCT02673359 (first received 3 February 2016).
Hezelgrave 2016 {published data only}
-
- Hezelgrave NL, Watson HA, Ridout A, Diab F, Seed PT, Chin‐Smith E, et al. Rationale and design of SuPPoRT: a multi‐centre randomised controlled trial to compare three treatments: cervical cerclage, cervical pessary and vaginal progesterone, for the prevention of preterm birth in women who develop a short cervix. BMC Pregnancy and Childbirth 2016;16(1):358. - PMC - PubMed
McLindon 2011 {published data only}
-
- McLindon L. In pregnant women with previous subfertility, does progesterone supplementation decrease the likelihood of miscarriage?. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336812 (first received 18 April 2011).
Rodger 2017 {published data only}
-
- Rodger M. A pilot study assessing the feasibility of a randomized controlled trial evaluating aspirin versus low‐molecular‐weight heparin (LMWH) and aspirin in women with antiphospholipid syndrome and pregnancy loss. clinicaltrials.gov/ct2/show/NCT03100123 (4 April 2017).
Schreiber 2017 {published data only}
-
- Schreiber K, Breen K, Robinson SE, Hunt BJ, Jacobsen S, Cohen H, et al. Hydroxychloroquine to improve pregnancy outcome in women with antiphospholipid antibodies (HYPATIA) protocol: a multinational randomized controlled trial of hydroxychloroquine versus placebo in addition to standard treatment in pregnant women with antiphospholipid syndrome or antibodies. Seminars in Thrombosis and Hemostasis 2017;43(6):562‐71. - PubMed
Additional references
Askie 2007
-
- Askie LM, Duley L, Henderson‐Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre‐eclampsia: a meta‐analysis of individual patient data. Lancet 2007;369(9575):1791‐8. - PubMed
Baibazarova 2013
-
- Baibazarova E, Beek C, Cohen‐Kettenis PT, Buitelaar J, Shelton KH, Goozen SH. Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology 2013;38(6):907‐15. - PubMed
Black 2008a
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. - PubMed
Black 2008b
-
- Black M, Shetty A, Bhattacharya S. Obstetric outcomes subsequent to intrauterine death in the first pregnancy. BJOG: an international journal of obstetrics and gynaecology 2008;115(2):269‐74. - PubMed
Bujold 2010
-
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta‐analysis. Obstetrics and Gynecology 2010;116(2):402‐14. - PubMed
De Jong 2014
Duffett 2015
-
- Duffett L, Rodger M. LMWH to prevent placenta‐mediated pregnancy complications: an update. British Journal of Haematology 2015;168(5):619‐38. - PubMed
Duley 2007
Dunkel Schetter 2011
-
- Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology 2011;62:531‐58. - PubMed
Flenady 2011
-
- Flenady V, Koopmans L, Middleton P, Froen JF, Smith, GC, Gibbons K, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet 2011;377(9774):1331–40. - PubMed
Flenady 2015
-
- Flenady V. Epidemiology of fetal and neonatal pathology. In: Khong TY, Malcomson RDG editor(s). Keeling's Fetal and Neonatal Pathology. 1. Springer, Cham, 2015.
Flenady 2016
-
- Flenady V, Wojcieszek AM, Middleton P, Ellwood D, Erwich, J, Coory M, et al. Lancet Ending Preventable Stillbirths series Study Group. Stillbirths: recall to action in high‐income countries. Lancet 2016;387(10019):691‐702. - PubMed
Fockler 2017
-
- Fockler ME, Ladhani NN, Watson J, Barrett JF. Pregnancy subsequent to stillbirth: medical and psychosocial aspects of care. Seminars in Fetal and Neonatal Medicine 2017;22(3):186‐92. - PubMed
Getahun 2009
-
- Getahun D, Lawrence JM, Fassett MJ, Strickland D, Koebnick C, Chen W, et al. The association between stillbirth in the first pregnancy and subsequent adverse perinatal outcomes. American Journal of Obstetrics and Gynecology 2009;201(4):378. - PubMed
Goldenberg 2016
-
- Goldenberg RL, Saleem S, Pasha O, Harrison MS, McClure EM. Reducing stillbirths in low‐income countries. Acta Obstetricia et Gynecologica Scandinavica 2016;95(2):135‐43. - PubMed
Gravensteen 2018
Haas 2018
Heazell 2016
-
- Heazell AE, Siassakos D, Blencowe H, Bhutta ZA, Cacciatore J, Dang N, et al. Lancet Ending Preventable Stillbirths series Study Group. Stillbirths: economic and psychosocial consequences. Lancet 2016;387(10018):604‐16. - PubMed
Heinonen 2000
-
- Heinonen S, Kirkinen P. Pregnancy outcome after previous stillbirth resulting from causes other than maternal conditions and fetal abnormalities. Birth 2000;27(1):33‐7. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 2001
-
- Hughes P, Turton P, Hopper E, McGauley GA, Fonagy P. Disorganised attachment behaviour among infants born subsequent to stillbirth. Journal of Child Psychology and Psychiatry and Allied Disciplines 2001;42(6):791‐801. - PubMed
Hussey 2007
-
- Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemporary Clinical Trials 2007;28(2):182‐91. - PubMed
Lamont 2015
-
- Lamont K, Scott NW, Jones GT, Bhattacharya S. Risk of recurrent stillbirth: systematic review and meta‐analysis. BMJ 2015;350:h3080. - PubMed
Lawn 2016
-
- Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors and potential for progress towards 2030. Lancet 2016;387(10018):587‐603. - PubMed
Lee 2017
-
- Lee L, McKenzie‐McHarg K, Horsch A. The impact of miscarriage and stillbirth on maternal–fetal relationships: an integrative review. Journal of Reproductive and Infant Psychology 2017;35(1):32‐52. - PubMed
Liberati 2009
McClure 2018
-
- McClure EM, Garces A, Saleem S, Moore JL, Bose CL, Esamai F, et al. Global Network for Women's and Children's Health Research: probable causes of stillbirth in low‐ and middle‐income countries using a prospectively defined classification system. BJOG: an international journal of obstetrics and gynaecology 2018;125(2):131‐8. - PMC - PubMed
Mdege 2011
-
- Mdege ND, Man M‐S, Taylor CA, Torgerson DJ. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. Journal of Clinical Epidemiology 2011;64(9):936‐48. - PubMed
Meaney 2017
Meredith 2017
Mills 2014
-
- Mills TA, Ricklesford C, Cooke A, Heazell AE, Whitworth M, Lavender T. Parents' experiences and expectations of care in pregnancy after stillbirth or neonatal death: a metasynthesis. BJOG: an international journal of obstetrics and gynaecology 2014;121(8):943‐50. - PubMed
Mills 2016
Monari 2010
-
- Monari F, Facchinetti F. Management of subsequent pregnancy after antepartum stillbirth. A review. Journal of Maternal‐fetal & Neonatal Medicine 2010;23(10):1073‐84. - PubMed
Monari 2016
O'Donnell 2009
-
- O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Developmental Neuroscience 2009;31(4):285‐92. - PubMed
Ogwulu 2015
Onwude 2006
-
- Onwude JL, Eisman V, Selo‐Ojeme DO. Recurrent stillbirths: a matched case‐control study of unexplained stillbirths at term. Journal of Obstetrics and Gynaecology 2006;26(3):205‐7. - PubMed
Paull 2013
-
- Paull C, Robson S. After stillbirth, what next?. O&G Magazine 2013; Vol. 15, issue 4:579.
Reddy 2007
-
- Reddy UM. Prediction and prevention of recurrent stillbirth. Obstetrics and Gynecology 2007;110(5):1151‐64. - PubMed
Reinebrant 2018
-
- Reinebrant HE, Leisher SH, Coory M, Henry S, Wojcieszek AM, Gardener G, et al. Making stillbirths visible: a systematic review of globally reported causes of stillbirth [2018]. BJOG: an international journal of obstetrics and gynaecology 2018;125(2):212‐24. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberge 2013
-
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low‐dose aspirin: a meta‐analysis. Ultrasound in Obstetrics & Gynecology 2013;41(5):491‐9. - PubMed
Roberge 2016
-
- Roberge S, Odibo AO, Bujold E. Aspirin for the prevention of preeclampsia and Intrauterine growth restriction. Clinics in Laboratory Medicine 2016;36(2):319‐29. - PubMed
Robson 2001
-
- Robson S, Chan A, Keane RJ, Luke CG. Subsequent birth outcomes after an unexplained stillbirth: preliminary population‐based retrospective cohort study. Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(1):29‐35. - PubMed
Robson 2006
-
- Robson S, Thompson J, Ellwood D. Obstetric management of the next pregnancy after an unexplained stillbirth: an anonymous postal survey of Australian obstetricians. Australian & New Zealand Journal of Obstetrics & Gynaecology 2006;46(4):278‐81. - PubMed
Robson 2009
-
- Robson SJ, Leader LR, Dear KBG, Bennett MJ. Women’s expectations of management in their next pregnancy after an unexplained stillbirth: an Internet‐based empirical study. Australian & New Zealand Journal of Obstetrics & Gynaecology 2009;49(6):642‐6. - PubMed
Robson 2010
-
- Robson SJ, Leader LR. Management of subsequent pregnancy after an unexplained stillbirth. Journal of Perinatology 2010;30(5):305‐10. - PubMed
Rodger 2016
-
- Rodger MA, Gris J‐C, Vries JI, Martinelli I, Rey É, Schleussner E, et al. Low‐molecular‐weight heparin and recurrent placenta‐mediated pregnancy complications: a meta‐analysis of individual patient data from randomised controlled trials. Lancet 2016;388(10060):2629‐41. - PubMed
Saade 2011
-
- Saade G. Management of the subsequent pregnancy. Stillbirth: Prediction, Prevention and Management. 1st Edition. Wiley‐Blackwell, 2011.
Saccone 2017
-
- Saccone G, Schoen C, Franasiak JM, Scott RT Jr, Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta‐analysis of randomized, controlled trials. Fertility and Sterility 2017;107(2):430‐8.e3. - PubMed
San Lazaro 2017
Scheres 2017
Silver 2011
-
- Silver RM, Branch DW, Goldenberg R, Iams JD, Klebanoff MA. Nomenclature for pregnancy outcomes: time for a change. Obstetrics and Gynecology 2011;118(6):1402‐8. - PubMed
Simmons 2011
-
- Simmons HA, Goldberg LS. 'High‐risk' pregnancy after perinatal loss: understanding the label. Midwifery 2011;27(4):452‐7. - PubMed
Skeith 2016
-
- Skeith L, Carrier M, Kaaja R, Martinelli I, Petroff D, Schleussner E, et al. A meta‐analysis of low‐molecular‐weight heparin to prevent pregnancy loss in women with inherited thrombophilia. Blood 2016;127(13):1650‐5. - PubMed
Su 2015
-
- Su Q, Zhang H, Zhang Y, Zhang H, Ding D, Zeng J, et al. Maternal stress in gestation: Birth outcomes and stress‐related hormone response of the neonates. Pediatrics and Neonatology 2015;56(6):376–81. - PubMed
Van den Bergh 2005
-
- Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neuroscience and Biobehavioral Reviews 2005;29(2):237‐58. - PubMed
Wojcieszek 2018
-
- Wojcieszek AM, Boyle FM, Belizán JM, Cassidy J, Cassidy P, Erwich J, et al. Care in subsequent pregnancies following stillbirth: an international survey of parents. BJOG: an international journal of obstetrics and gynaecology 2018;125(2):193‐201. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

