Six-gene Assay as a new biomarker in the blood of patients with colorectal cancer: establishment and clinical validation
- PMID: 30556647
- PMCID: PMC6441906
- DOI: 10.1002/1878-0261.12427
Six-gene Assay as a new biomarker in the blood of patients with colorectal cancer: establishment and clinical validation
Abstract
Colorectal cancer (CRC) is the second most common cancer in men and the third most common cancer in women. Although long-term survival has improved over the past 30 years, at least 50% of patients with CRC will develop metastases after diagnosis. In this study, we examined whether quantifying the mRNA of six CRC-related genes in the blood could improve disease assessment through detection of circulating tumor cells (CTC), and thereby improve progression prediction in relapsed CRC patients. Cell spiking assay and RT-PCR were performed with blood samples from healthy volunteers spiked with six CRC cell lines to generate an algorithm, herein called the Six-gene Assay, based on six genes (CEA, EpCAM, CK19, MUC1, EGFR and C-Met) for CTC detection. The CTCs of 50 relapsed CRC patients were then respectively measured by CEA Gene Assay (single-gene assay control) and Six-gene Assay. Subsequently, receiver operating characteristic analysis of the CTC panel performance in diagnosing CRC was conducted for both assays. Moreover, the 2-year progression-free survival (PFS) of all patients was collected, and the application of CEA Gene Assay and Six-gene Assay in predicting PFS was carefully evaluated with different CTC cutoff values. Encouragingly, we successfully constructed the first multiple gene-based algorithm, named the Six-gene Assay, for CTC detection in CRC patients. Six-gene Assay was more sensitive than CEA Gene Assay; for instance, in 50 CRC patients, the positive rate of Six-gene Assay in CTC detection was 82%, whereas that of CEA Gene Assay was only 70%. Moreover, Six-gene Assay was more sensitive and accurate than CEA Gene Assay in diagnosing CRC as well as predicting the 2-year PFS of CRC patients. Statistical analysis demonstrated that CTC numbers measured by Six-gene Assay were significantly associated with 2-year PFS. This novel Six-gene Assay improves the definition of disease status and correlates with PFS in relapsed CRC, and thus holds promise for future clinical applications.
Keywords: RT-PCR; Six-gene Assay; biomarker; circulating tumor cells; colorectal cancer.
© 2018 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


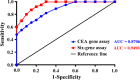
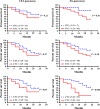
References
-
- Bardelli A and Pantel K (2017) Liquid biopsies, what we do not know (yet). Cancer Cell 31, 172–179. - PubMed
-
- Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E, Schumacher U, Costes V, Maudelonde T, Pantel K et al (2015) Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 75, 892–901. - PubMed
-
- Chaffer CL and Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331, 1559–1564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PWZz2013-06/Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai/International
- 16ZR1431400/Shanghai Science and Technology Commission Foundation/International
- ZJZLSYS001/Open Foundation from Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province/International
- 2017C33047/Science Technology Research Program of Zhejiang Province/International
- 21574118/National Natural Science Foundation of China/International
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

