Short-acting insulin analogues versus regular human insulin for adult, non-pregnant persons with type 2 diabetes mellitus
- PMID: 30556900
- PMCID: PMC6517032
- DOI: 10.1002/14651858.CD013228
Short-acting insulin analogues versus regular human insulin for adult, non-pregnant persons with type 2 diabetes mellitus
Abstract
Background: The use of short-acting insulin analogues (insulin lispro, insulin aspart, insulin glulisine) for adult, non-pregnant people with type 2 diabetes is still controversial, as reflected in many scientific debates.
Objectives: To assess the effects of short-acting insulin analogues compared to regular human insulin in adult, non-pregnant people with type 2 diabetes mellitus.
Search methods: For this update we searched CENTRAL, MEDLINE, Embase, the WHO ICTRP Search Portal, and ClinicalTrials.gov to 31 October 2018. We placed no restrictions on the language of publication.
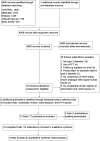
Selection criteria: We included all randomised controlled trials with an intervention duration of at least 24 weeks that compared short-acting insulin analogues to regular human insulin in the treatment of people with type 2 diabetes, who were not pregnant.
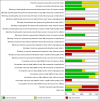
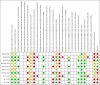
Data collection and analysis: Two review authors independently extracted data and assessed the risk of bias. We assessed dichotomous outcomes by risk ratios (RR), and Peto odds ratios (POR), with 95% confidence intervals (CI). We assessed continuous outcomes by mean differences (MD) with 95% CI. We assessed trials for certainty of the evidence using the GRADE approach.
Main results: We identified 10 trials that fulfilled the inclusion criteria, randomising 2751 participants; 1388 participants were randomised to receive insulin analogues and 1363 participants to receive regular human insulin. The duration of the intervention ranged from 24 to 104 weeks, with a mean of about 41 weeks. The trial populations showed diversity in disease duration, and inclusion and exclusion criteria. None of the trials were blinded, so the risk of performance bias and detection bias, especially for subjective outcomes, such as hypoglycaemia, was high in nine of 10 trials from which we extracted data. Several trials showed inconsistencies in the reporting of methods and results.None of the included trials defined all-cause mortality as a primary outcome. Six trials provided Information on the number of participants who died during the trial, with five deaths out of 1272 participants (0.4%) in the insulin analogue groups and three deaths out of 1247 participants (0.2%) in the regular human insulin groups (Peto OR 1.66, 95% CI 0.41 to 6.64; P = 0.48; moderate-certainty evidence). Six trials, with 2509 participants, assessed severe hypoglycaemia differently, therefore, we could not summarise the results with a meta-analysis. Overall, the incidence of severe hypoglycaemic events was low, and none of the trials showed a clear difference between the two intervention arms (low-certainty evidence).The MD in glycosylated haemoglobin A1c (HbA1c) change was -0.03% (95% CI -0.16 to 0.09; P = 0.60; 9 trials, 2608 participants; low-certainty evidence). The 95% prediction ranged between -0.31% and 0.25%. The MD in the overall number of non-severe hypoglycaemic episodes per participant per month was 0.08 events (95% CI 0.00 to 0.16; P = 0.05; 7 trials, 2667 participants; very low-certainty evidence). The 95% prediction interval ranged between -0.03 and 0.19 events per participant per month. The results provided for nocturnal hypoglycaemic episodes were of questionable validity. Overall, there was no clear difference between the two short-acting insulin analogues and regular human insulin. Two trials assessed health-related quality of life and treatment satisfaction, but we considered the results for both outcomes to be unreliable (very low-certainty evidence).No trial was designed to investigate possible long term effects (all-cause mortality, microvascular or macrovascular complications of diabetes), especially in participants with diabetes-related complications. No trial reported on socioeconomic effects.
Authors' conclusions: Our analysis found no clear benefits of short-acting insulin analogues over regular human insulin in people with type 2 diabetes. Overall, the certainty of the evidence was poor and results on patient-relevant outcomes, like all-cause mortality, microvascular or macrovascular complications and severe hypoglycaemic episodes were sparse. Long-term efficacy and safety data are needed to draw conclusions about the effects of short-acting insulin analogues on patient-relevant outcomes.
Conflict of interest statement
BF: none known.
AS: was involved in the preparation of a report on the effects of long‐acting insulin analogues versus other basal insulins in the therapy of patients with type 1 and type 2 diabetes mellitus for IQWiG, the German Institute for Quality and Efficiency in Health Care.
KJ: was involved in the preparation of the reports on short‐acting insulin analogues for the treatment of diabetes mellitus for the Institute for Quality and Efficiency in Health Care.
KH: has received payment for lectures, travel/accommodations/meeting expenses and consultancy from various sources (Novo Nordisk, Novartis, Medtronic, Eli Lilly, Sanofi Aventis, Merck Sharp & Dohme, AstraZeneca).
TS: none known.
AB: none known.
FMG: none known.
Figures








Comment in
-
Review: Rapid-acting analogues do not differ from regular human insulin for mortality or HbA1c in type 2 diabetes.Ann Intern Med. 2019 Apr 16;170(8):JC39. doi: 10.7326/ACPJ201904160-039. Ann Intern Med. 2019. PMID: 30986829 No abstract available.
References
References to studies included in this review
-
- Altuntas Y, Ozen B, Ozturk B, Sengul A, Ucak S, Ersoy O, et al. Comparison of additional metformin or NPH insulin to mealtime insulin lispro therapy with mealtime human insulin therapy in secondary OAD failure. Diabetes, Obesity and Metabolism 2003;5(6):371‐8. [PUBMED: 14617222] - PubMed
- IQWiG (Institute for Quality and Efficiency in Health Care). Short‐acting insulin analogues in the treatment of diabetes mellitus type 2. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG); 2005 December. Abschlusbericht A05‐04.
-
- Bastyr EJ III, Huang Y, Brunelle RL, Vignati L, Cox DJ, Kotsanos JG. Factors associated with nocturnal hypoglycaemia among patients with type 2 diabetes new to insulin therapy: experience with insulin Lispro. Diabetes Obesity and Metabolism 2000;2(1):39‐46. [PUBMED: 11220353] - PubMed
- IQWiG (Institute for Quality and Efficiency in Health Care). Short‐acting insulin analogues in the treatment of diabetes mellitus type 2. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG); 2005 December. Abschlusbericht A05‐04.
-
- Dailey G, Rosenstock J, Moses RG, Ways K. Insulin Glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 2004;27(10):2363‐8. [PUBMED: 15451901] - PubMed
- IQWiG (Institute for Quality and Efficiency in Health Care). Short‐acting insulin analogues in the treatment of diabetes mellitus type 2. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG); 2005 December. Abschlusbericht A05‐04.
-
- Hermann BL, Kasser C, Keuthage W, Huptas M, Dette H, Klute A. Comparison of insulin Aspart vs. regular human insulin with or without insulin Detemir concerning adipozytokines and metabolic effects in patients with type 2 diabetes mellitus. Experimental & Clinical Endocrinology & Diabetes 2013;121(4):210‐3. [PUBMED: 23512415] - PubMed
-
- NCT01650129. Safety and efficacy of biphasic insulin Aspart 50 in subjects with type 2 diabetes. clinicaltrials.gov/ct2/show/NCT01650129 (first posted July 26, 2012).
- Novo Nordisk. An open‐labelled randomised, parallel group, multicentre, safety and efficacy study of NN‐X14Mix50 (BIAsp50) in a twice daily regimen in type 2 diabetic subjects. novonordisk‐trials.com (accessed 4 December 2018).
References to studies excluded from this review
-
- Bi YF, Zhao LB, Li XY, Wang WQ, Sun SY, Chen YH, et al. A 2‐way cross‐over, open‐labeled trial to compare efficacy and safety of insulin Aspart and Novolin R delivered with CSII in 21 Chinese diabetic patients. Chinese Medical Journal 2007;120(19):1700‐3. [PUBMED: 17935674] - PubMed
-
- Boehm BO, Vaz JA, Bronsted L, Home PD. Long‐term efficacy and safety of biphasic insulin Aspart in patients with type 2 diabetes. European Journal of Internal Medicine 2004;15(8):496‐502. [PUBMED: 15668084] - PubMed
-
- Boivin S, Belicar P, Melki V. Assessment of in vivo stability of a new insulin preparation for implantable insulin pumps. A randomized multicenter prospective trial. Diabetes Care 1999;22(12):2089‐90. [PUBMED: 10587853] - PubMed
-
- Bott U, Ebrahim S, Hirschberger S, Skovlund SE. Effect of the rapid‐acting insulin analogue insulin Aspart on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabetic Medicine 2003;20:626–34. [PUBMED: 12873289] - PubMed
-
- Caixàs A, Pérez A, Payés A, Otal C, Carreras G, Ordóñez‐Llanos J, et al. Effects of a short‐acting insulin analog (insulin Lispro) versus regular insulin on lipid metabolism in insulin‐dependent diabetes mellitus. Metabolism: Clinical & Experimental 1998;47(4):371‐6. [PUBMED: 9580247] - PubMed
References to studies awaiting assessment
-
- Farshchi A, Aghili R, Oskuee M, Rashed M, Noshad S, Kebriaeezadeh A, et al. Biphasic insulin Aspart 30 vs. NPH plus regular human insulin in type 2 diabetes patients; a cost‐effectiveness study. BMC Endocrine Disorders 2016;16(1):35. [PUBMED: 27278922] - PMC - PubMed
- NCT01889095. Biphasic insulin Aspart versus NPH plus regular human insulin in type 2 diabetic patients. clinicaltrials.gov/ct2/show/NCT01889095 (first posted 23 June 2013).
-
- NCT01500850. Establishing cardiovascular biomarkers to define preferred Lantus® use. clinicaltrials.gov/ct2/show/NCT01500850 (first posted 29 December 2011).
Additional references
-
- American Diabetes Association (ADA). Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183‐97. - PubMed
-
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl 1):S5‐20. - PubMed
-
- American Diabetes Association (ADA). Standards of medical care in diabetes ‐ 2017. Diabetes Care 2017;40(Supplement 1):S 11‐33.
-
- American Diabetes Association (ADA). Standards of medical care in diabetes ‐ 2018. Diabetes Care 2018;41(Suppl 1):S1‐S159.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

