Systemic Tofacitinib Concentrations in Adult Patients With Atopic Dermatitis Treated With 2% Tofacitinib Ointment and Application to Pediatric Study Planning
- PMID: 30556911
- PMCID: PMC6590358
- DOI: 10.1002/jcph.1360
Systemic Tofacitinib Concentrations in Adult Patients With Atopic Dermatitis Treated With 2% Tofacitinib Ointment and Application to Pediatric Study Planning
Abstract
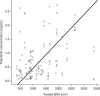
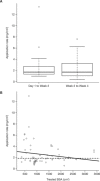
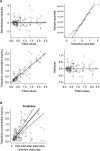
Atopic dermatitis is a chronic eczematous, pruritic, inflammatory skin condition affecting children and adults. Tofacitinib is a Janus kinase inhibitor. The efficacy, safety, and pharmacokinetics of 2% tofacitinib ointment twice daily have been evaluated in a 4-week phase 2a multisite randomized, double-blind, vehicle-controlled, parallel-group study (NCT02001181) in adult patients with mild to moderate atopic dermatitis and 2% to 20% body surface area (BSA) involvement. Tofacitinib ointment demonstrated significantly greater efficacy versus vehicle for all efficacy end points and had an acceptable safety profile. Predose and postdose pharmacokinetic samples were collected in week 2 and week 4. The objective of this analysis was to assess if predicted mean tofacitinib concentrations with topical application at higher treated BSA across age groups would exceed relevant concentration thresholds based on oral doses of tofacitinib. In this analysis, the pharmacokinetic concentrations were characterized using a linear mixed-effects model. The model was used to predict concentrations for adults with higher (>20%) treatable BSA. Adult concentrations were used to extrapolate concentrations to a pediatric population (2 to 17 years) using allometric principles. The predicted systemic concentrations for 2% tofacitinib ointment in both adult and pediatric populations at treated BSA ≤50% for a mild to moderate atopic dermatitis population did not exceed those reported for the 10th percentile of observed oral tofacitinib 5-mg twice-daily doses in patients with moderate to severe plaque psoriasis. The methodology described will enable analysis and prediction of systemic concentrations for topical agents.
Keywords: atopic dermatitis; pharmacokinetics; systemic concentration; tofacitinib; topical.
© 2018, The Authors. The Journal of Clinical Pharmacology published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Figures
References
-
- DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc. 2012;33:227–234. - PubMed
-
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980;92(suppl):44–47.
-
- Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69:3–16. - PubMed
-
- Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192–199. - PubMed
-
- Weisshaar E, Diepgen TL, Bruckner T, et al. Itch intensity evaluated in the German Atopic Dermatitis Intervention Study (GADIS): correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm Venereol. 2008;88:234–239. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

