PR3-ANCA and panel diagnostics in pediatric inflammatory bowel disease to distinguish ulcerative colitis from Crohn's disease
- PMID: 30557305
- PMCID: PMC6296712
- DOI: 10.1371/journal.pone.0208974
PR3-ANCA and panel diagnostics in pediatric inflammatory bowel disease to distinguish ulcerative colitis from Crohn's disease
Abstract
Background: Accurate classification of patients with inflammatory bowel disease into the subtypes ulcerative colitis (UC) and Crohn's disease (CD) is still a challenge, but important for therapy and prognosis.
Objectives: To evaluate the diagnostic utility of anti-neutrophil cytoplasmic antibodies specific for proteinase-3 (PR3-ANCA) for ulcerative colitis (UC) and the value of an antibody panel incorporating PR3-ANCA to differentiate between Crohn's disease (CD) and UC.
Study design: In this cohort study, 122 pediatric and adolescent individuals were retrospectively included (61 IBD patients of two clinical centers, 61 non-IBD controls). All subjects had a comprehensive antibody profile done from stored sera taken close to time of diagnosis. By employing quasi-exhaustive logistic regression the best discriminative model for UC and CD,subjects was determined in a training cohort and confirmed in a validation cohort.
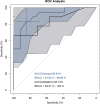
Results: PR3-ANCA was specifically associated with UC (odds ratio (OR), 17.6; 95% confidence interval (CI); 3.6, 87); P < .001). A four antibody-panel including PR3-ANCA had an AUC of 90.81% (95%CI; 81.93, 99.69) to distinguish between UC and CD in the training cohort. In a smaller external validation cohort, the AUC was 84.13% (95%CI; 64.21, 100) for accurate diagnosis of CD and UC.
Conclusion: PR3-ANCA is highly specific for UC. The differentiating capability of a panel, which contains PR3-ANCA and weighs broadly available antibodies, is superior and utilization of the panel can support accurate classification in the work-up of pediatric and adolescent patients with IBD patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Clinical Validity of Anti-Proteinase 3 Antibodies in Patients with Inflammatory Bowel Disease: A Short Meta-Analysis.Diagnostics (Basel). 2023 Dec 16;13(24):3682. doi: 10.3390/diagnostics13243682. Diagnostics (Basel). 2023. PMID: 38132266 Free PMC article. Review.
-
The diagnostic role and clinical association of serum proteinase 3 anti-neutrophil cytoplasmic antibodies in Chinese patients with inflammatory bowel disease.Scand J Gastroenterol. 2020 Jul;55(7):806-813. doi: 10.1080/00365521.2020.1781926. Epub 2020 Jun 22. Scand J Gastroenterol. 2020. PMID: 32568566
-
PR3-ANCA: a promising biomarker for ulcerative colitis with extensive disease.Clin Chim Acta. 2013 Sep 23;424:267-73. doi: 10.1016/j.cca.2013.06.005. Epub 2013 Jun 24. Clin Chim Acta. 2013. PMID: 23806819
-
Antineutrophil cytoplasmic antibodies in children with inflammatory bowel disease: prevalence and diagnostic value.J Pediatr Gastroenterol Nutr. 1997 Aug;25(2):142-8. doi: 10.1097/00005176-199708000-00003. J Pediatr Gastroenterol Nutr. 1997. PMID: 9252899
-
Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America.J Pediatr Gastroenterol Nutr. 2007 May;44(5):653-74. doi: 10.1097/MPG.0b013e31805563f3. J Pediatr Gastroenterol Nutr. 2007. PMID: 17460505
Cited by
-
Clinical Validity of Anti-Proteinase 3 Antibodies in Patients with Inflammatory Bowel Disease: A Short Meta-Analysis.Diagnostics (Basel). 2023 Dec 16;13(24):3682. doi: 10.3390/diagnostics13243682. Diagnostics (Basel). 2023. PMID: 38132266 Free PMC article. Review.
-
Correlation between Antibodies to Bacterial Lipopolysaccharides and Barrier Proteins in Sera Positive for ASCA and ANCA.Int J Mol Sci. 2020 Feb 18;21(4):1381. doi: 10.3390/ijms21041381. Int J Mol Sci. 2020. PMID: 32085663 Free PMC article.
-
Identification of Potential Functional Modules and Diagnostic Genes for Crohn's Disease Based on Weighted Gene Co-expression Network Analysis and LASSO Algorithm.Turk J Gastroenterol. 2025 Jan 6;36(4):209-218. doi: 10.5152/tjg.2025.23605. Turk J Gastroenterol. 2025. PMID: 40181741 Free PMC article.
-
The search for the Holy Grail: autoantigenic targets in primary sclerosing cholangitis associated with disease phenotype and neoplasia.Auto Immun Highlights. 2020 Mar 16;11(1):6. doi: 10.1186/s13317-020-00129-x. Auto Immun Highlights. 2020. PMID: 32178720 Free PMC article. Review.
-
Inflammatory Bowel Disease and Its Association With Perinuclear Antineutrophil Cytoplasmic Antibodies: A Systematic Review.Cureus. 2024 Apr 8;16(4):e57872. doi: 10.7759/cureus.57872. eCollection 2024 Apr. Cureus. 2024. PMID: 38725759 Free PMC article. Review.
References
-
- Levine A, de Bie CI, Turner D, Cucchiara S, Sladek M, Murphy MS, et al. (2013) Atypical disease phenotypes in pediatric ulcerative colitis: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis 19: 370–377. 10.1002/ibd.23013 - DOI - PubMed
-
- Carvalho RS, Abadom V, Dilworth HP, Thompson R, Oliva-Hemker M, Cuffari C (2006) Indeterminate colitis: a significant subgroup of pediatric IBD. Inflamm Bowel Dis 12: 258–262. 10.1097/01.MIB.0000215093.62245.b9 - DOI - PubMed
-
- Matsui T, Yao T, Sakurai T, Yao K, Hirai F, Matake H, et al. (2003) Clinical features and pattern of indeterminate colitis: Crohn's disease with ulcerative colitis-like clinical presentation. J Gastroenterol 38: 647–655. 10.1007/s00535-003-1117-8 - DOI - PubMed
-
- Alexander F, Sarigol S, DiFiore J, Stallion A, Cotman K, Clark H, et al. (2003) Fate of the pouch in 151 pediatric patients after ileal pouch anal anastomosis. J Pediatr Surg 38: 78–82. 10.1053/jpsu.2003.50015 - DOI - PubMed
-
- Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. (2014) ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 58: 795–806. 10.1097/MPG.0000000000000239 - DOI - PubMed