Small-molecule induction of Aβ-42 peptide production in human cerebral organoids to model Alzheimer's disease associated phenotypes
- PMID: 30557391
- PMCID: PMC6296660
- DOI: 10.1371/journal.pone.0209150
Small-molecule induction of Aβ-42 peptide production in human cerebral organoids to model Alzheimer's disease associated phenotypes
Abstract
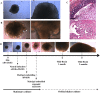
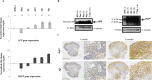
Human mini-brains (MB) are cerebral organoids that recapitulate in part the complexity of the human brain in a unique three-dimensional in vitro model, yielding discrete brain regions reminiscent of the cerebral cortex. Specific proteins linked to neurodegenerative disorders are physiologically expressed in MBs, such as APP-derived amyloids (Aβ), whose physiological and pathological roles and interactions with other proteins are not well established in humans. Here, we demonstrate that neuroectodermal organoids can be used to study the Aβ accumulation implicated in Alzheimer's disease (AD). To enhance the process of protein secretion and accumulation, we adopted a chemical strategy of induction to modulate post-translational pathways of APP using an Amyloid-β Forty-Two Inducer named Aftin-5. Secreted, soluble Aβ fragment concentrations were analyzed in MB-conditioned media. An increase in the Aβ42 fragment secretion was observed as was an increased Aβ42/Aβ40 ratio after drug treatment, which is consistent with the pathological-like phenotypes described in vivo in transgenic animal models and in vitro in induced pluripotent stem cell-derived neural cultures obtained from AD patients. Notably in this context we observe time-dependent Aβ accumulation, which differs from protein accumulation occurring after treatment. We show that mini-brains obtained from a non-AD control cell line are responsive to chemical compound induction, producing a shift of physiological Aβ concentrations, suggesting that this model can be used to identify environmental agents that may initiate the cascade of events ultimately leading to sporadic AD. Increases in both Aβ oligomers and their target, the cellular prion protein (PrPC), support the possibility of using MBs to further understand the pathophysiological role that underlies their interaction in a human model. Finally, the potential application of MBs for modeling age-associated phenotypes and the study of neurological disorders is confirmed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Kitazawa M, Medeiros R M. LaFerla F. Transgenic Mouse Models of Alzheimer Disease: Developing a Better Model as a Tool for Therapeutic Interventions. Curr Pharm Des. 2012;18: 1131–1147. 10.2174/138161212799315786 - DOI - PMC - PubMed
-
- Cummings J. Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes. Clin Transl Sci. 2018;11: 147–152. 10.1111/cts.12491 - DOI - PMC - PubMed
-
- Lesné S, Ming TK, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440: 352–357. 10.1038/nature04533 - DOI - PubMed
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β -peptide. Nat Rev Mol Cell Biol. 2007;8: 101–112. 10.1038/nrm2101 - DOI - PubMed
-
- Cavanaugh SE. Animal models of Alzheimer disease: historical pitfalls and a path forward. ALTEX. 2014;31: 279–302. doi: 10.14573/altex.1310071 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

