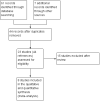
Recombinant growth hormone therapy for cystic fibrosis in children and young adults
- PMID: 30557452
- PMCID: PMC6517261
- DOI: 10.1002/14651858.CD008901.pub4
Recombinant growth hormone therapy for cystic fibrosis in children and young adults
Update in
-
Recombinant growth hormone therapy for cystic fibrosis in children and young adults.Cochrane Database Syst Rev. 2021 Aug 23;8(8):CD008901. doi: 10.1002/14651858.CD008901.pub5. Cochrane Database Syst Rev. 2021. PMID: 34424546 Free PMC article.
Abstract
Background: Cystic fibrosis (CF) is an inherited condition causing disease most noticeably in the lungs, digestive tract and pancreas. People with CF often have malnutrition and growth delay. Adequate nutritional supplementation does not improve growth optimally and hence an anabolic agent, recombinant human growth hormone (rhGH), has been proposed as a potential intervention. This is an update of a previously published review.
Objectives: To evaluate the effectiveness and safety of rhGH therapy in improving lung function, quality of life and clinical status of children and young adults with CF.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. Date of latest search: 22 October 2018.We also searched ongoing trials registers in clinicaltrials.gov from the United States and WHO International Clinical Trials Registry Platform (ICTRP). Date of latest search: 05 March 2018.We conducted a search of relevant endocrine journals and proceedings of the Endocrinology Society meetings using Web of Science, Scopus and Proceedings First. Date of latest search: 04 March 2018.
Selection criteria: Randomised and quasi-randomised controlled trials of all preparations of rhGH compared to either no treatment, or placebo, or each other at any dose (high-dose and low-dose) or route and for any duration, in children or young adults (aged up to 25 years) diagnosed with CF (by sweat test or genetic testing).
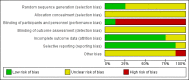
Data collection and analysis: Two authors independently screened papers, extracted trial details and assessed their risk of bias. We assessed the quality of the evidence using the GRADE system.
Main results: We included eight trials (291 participants, aged between five and 23 years) in this revision of the review. Seven trials compared standard-dose rhGH (approximately 0.3 mg/kg/week) to no treatment and one three-arm trial (63 participants) compared placebo, standard-dose rhGH (0.3 mg/kg/week) and high-dose rhGH (0.5 mg/kg/week). Six trials lasted for one year and two trials for six months. We found that rhGH treatment may improve some of the pulmonary function outcomes but there was no difference between standard and high-dose levels (low-quality evidence, limited by inconsistency across the trials, small number of participants and short duration of therapy). The trials show evidence of improvement in the anthropometric parameters (height, weight and lean body mass) with rhGH therapy, again no differences between dose levels. We found improvement in height for all comparisons (very low- to low quality evidence), but improvements in weight and lean body mass were only reported for standard-dose rhGH versus no treatment (very low-quality evidence). There is some evidence indicating a change in the level of fasting blood glucose with rhGH therapy, however, it did not cross the clinical threshold for diagnosis of diabetes in the trials of short duration (low-quality evidence). There is low- to very low-quality evidence for improvement of pulmonary exacerbations with no further significant adverse effects, but this is limited by the short duration of trials and the small number of participants. One small trial provided inconsistent evidence on improvement in quality of life (very low-quality evidence). There is limited evidence from three trials in improvements in exercise capacity (low-quality evidence). None of the trials have systematically compared the expense of therapy on overall healthcare costs.
Authors' conclusions: When compared with no treatment, rhGH therapy is effective in improving the intermediate outcomes in height, weight and lean body mass. Some measures of pulmonary function showed moderate improvement, but no consistent benefit was seen across all trials. The significant change in blood glucose levels, although not causing diabetes, emphasizes the need for careful monitoring of this adverse effect with therapy in a population predisposed to CF-related diabetes. No significant changes in quality of life, clinical status or side-effects were observed in this review due to the small number of participants. Long-term, well-designed randomised controlled trials of rhGH in individuals with CF are required prior to routine clinical use of rhGH in CF.
Conflict of interest statement
Melissa Putman declares she has performed consulting for Proteostasis Therapeutics, Inc. that was unrelated to this work.
The remaining authors declare that there are no financial conflicts of interest and they do not have any associations with any parties who may have vested interests in the results of this review.
Figures
Update of
-
Recombinant growth hormone therapy for cystic fibrosis in children and young adults.Cochrane Database Syst Rev. 2015 May 20;(5):CD008901. doi: 10.1002/14651858.CD008901.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Dec 17;12:CD008901. doi: 10.1002/14651858.CD008901.pub4. PMID: 25991406 Updated.
References
References to studies included in this review
Hardin 2001 {published data only}
-
- Hardin DS, Ellis K, McConnell RA, Seilheimer DK. Growth hormone improves lean body mass in prepubertal CF children. Pediatric Pulmonology 1998;26(Suppl 17):357. [CFGD Register: GN122a]
-
- Hardin DS, Ellis KJ, Dyson M, Rice J, McConnell R, Seilheimer DK. Growth hormone decreases protein catabolism in children with cystic fibrosis. Journal of Clinical Endocrinology and Metabolism 2001;86(9):4424‐8. [CFGD Register: GN122b] - PubMed
-
- Hardin DS, Ellis KJ, Dyson M, Rice J, McConnell R, et al. Growth hormone improves clinical status in prepubertal children with cystic fibrosis: results of a randomized controlled trial. Journal of Pediatrics 2001;139(5):636‐42. [CFGD Register: GN122c] - PubMed
-
- Hardin DS, Ellis KJ, McConnel R, Seilheimer DK. Growth hormone improves clinical status in cystic fibrosis children [abstract]. Pediatric Pulmonology 1999;28 (Suppl 19):297. [CFGD Register: GN264]
-
- NCT00256555. Growth Hormone Treatment Study in Children With Cystic Fibrosis. https://clinicaltrials.gov/ct2/show/NCT00256555 (first received 21 November 2005).
Hardin 2005a {published data only}
-
- Hardin DS, Rice J, Ahn C, Ferkol T, Howenstine M, et al. Growth hormone treatment enhances nutrition and growth in children with cystic fibrosis receiving enteral nutrition. Journal of Pediatrics 2005;146(3):324‐8. [CFGD Register: GN265d] - PubMed
Hardin 2005b {published data only}
-
- Hardin DS, Ahn C, Prestidge C, Seilheimer DK, Ellis KJ. Growth hormone improves bone mineral content in children with cystic fibrosis. Journal of Pediatric Endocrinology & Metabolism 2005;18(6):589‐95. [CFGD Register: GN122d] - PubMed
Hardin 2006 {published data only}
-
- Hardin D, Rice J, Ahn C, Brown D, Chatfield B, Dyson M, et al. Growth hormone improves bone mineralization in prepubertal children with CF ‐ results of a multicenter study. Pediatric Pulmonolgy 2004;38(Suppl 27):343. [CENTRAL: CN‐01400578; CFGD Register: GN265c; CRS: 6570051]
-
- Hardin DS, Adams‐Huet B, Brown D, Chatfield B, Dyson M, Ferkol T, et al. Online Supplement to 'Growth hormone treatment improves growth and clinical status in prepubertal children with cystic fibrosis: results of a multicenter randomized controlled trial'. [online]. Journal of Clinical Endocrinology and Metabolism 2006;91(12):4925‐9 Online. [CENTRAL: CN‐00593083; CFGD Register: GN265f; CRS: 1418647] - PubMed
-
- Hardin DS, Adams‐Huet B, Brown D, Chatfield B, Dyson M, et al. Growth hormone treatment improves growth and clinical status in prepubertal children with cystic fibrosis: results of a multicenter randomized controlled trial. Journal of Clinical Endocrinology and Metabolism 2006;91(12):4925‐9. [CFGD Register: GN265e] - PubMed
-
- Hardin DS, Chatfield B, Dyson M, Hicks D, Howenstine M, Lee P, et al. Multicentre trial of growth hormone in children with CF. Pediatric Pulmonology 2001;Suppl 22:338. [CENTRAL: CN‐00362183; CFGD Register: GN265a; CRS: 1231204]
-
- Hardin DS, Rice J, Ahn C, Chatfield B, Dyson M, Howenstein M, et al. Growth hormone improves pulmonary function, weight, and height ‐ results from a multicenter study [abstract]. Pediatric Pulmonology 2002;34 (Suppl 24):337. [CFGD Register: GN265b]
Hütler 2002 {published data only (unpublished sought but not used)}
-
- Hütler M, Schnabel D, Staab D, Tacke A, Wahn U, Boning D. Growth hormone enhances peak performance in cystic fibrosis. International Journal of Sports Medicine 1998;19(Suppl 1):S17. [CENTRAL: CN‐00494036; CFGD Register: GN125b; CRS: 1339962]
-
- Hütler M, Schnabel D, Staab D, Tacke A, Wahn U, et al. Effect of growth hormone on exercise tolerance in children with cystic fibrosis. Medicine and Science in Sports and Exercise 2002;34(4):567‐72. [CFGD Register: GN125a] - PubMed
Schibler 2003 {published data only}
-
- Schibler A, Heiden R, Birrer P, Mullis PE. Moderate improved exercise capacity in patients with cystic fibrosis after treatment with recombinant human growth hormone [abstract]. Proceedings of the 12th European Respiratory Society Annual Congress; 2002 Sept 14‐18; Stockholm. 2002:P3287. [GN121b]
-
- Heiden R, Kraemer R, Birrer P, Waldegg G, Mullis PE. Effect of growth hormone (r‐hGH) treatment on working capacity, body composition, lung function and immunological parameters in patients with cystic fibrosis (CF) [abstract]. Proceedings of the 21st European Cystic Fibrosis Conference; 1997 June 1‐6; Davos, Switzerland. 1997:132. [GN121a]
Schnabel 2007 {published data only}
-
- Grasemann C, Ratjen F, Schnabel D, Reutershahn E, Vester U, Grasemann H. Effect of growth hormone therapy on nitric oxide formation in cystic fibrosis patients. European Respiratory Journal 2008;31(4):815‐21. [CFGD Register: GN127e] - PubMed
-
- Grasemann H, Grasemann C, Schnabel D, Ratjen F. Growth hormone therapy results in increased L‐Arginine and nitrate concentrations in serum but decreased exhaled oxide in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 2006;41 (Suppl 29):335. [CFGD Register: GN127b]
-
- Grasemann H, Grasemann C, Schnabel D, Ratjen F. Recombinant human growth hormone therapy results in increased systemic nitric oxide (NO) formation but decreased exhaled NO in patients with cystic fibrosis [abstract]. Proceedings of the American Thoracic Society International Conference; 2006 May 19‐24; California, USA. 2006:A408. [CFGD Register: GN127a]
-
- Schnabel D, Grasemann C, Staab D, Wollmann H, Ratjen F. A multicenter, randomized, double‐blind placebo‐controlled trial evaluating the metabolic and respiratory effects of growth hormone in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2006;41 (Suppl 29):393. [CFGD Register: GN127c] - PubMed
-
- Schnabel D, Grasemann C, Staab D, Wollmann H, Ratjen F. A multicenter, randomized, double‐blind, placebo‐controlled trial to evaluate the metabolic and respiratory effects of growth hormone in children with cystic fibrosis. Pediatrics 2007;119(6):e1230‐8. [CFGD Register: GN127d] - PubMed
Stalvey 2012 {published data only}
-
- Stalvey MS, Geller DE, Anbar RD, Konstan MW, Jacobs JR, Bakker B. Growth hormone (GH) increases height, weight and lean body mass (LBM) in prepubertal children with cystic fibrosis (CF): results of a multicenter randomized control trial [abstract]. Pediatric Pulmonology 2007;42 (Suppl 30):393. [CFGD Register: GN128a]
References to studies excluded from this review
Alemzadeh 1998 {published data only}
-
- Alemzadeh R, Upchurch L, McCarthy V. Anabolic effects of growth hormone treatment in young children with cystic fibrosis. Journal of the American College of Nutrition 1998;17(5):419‐24. [PUBMED: 9791837] - PubMed
Bucuvalas 2001 {published data only}
-
- Bucuvalas JC, Chernausek SD, Alfaro MP, Krug S, Ritschel W, Wilmott RW. Insulin‐like growth factor ‐I enhances linear growth in undernourished prepubertal children with cystic fibrosis [abstract]. Pediatric Pulmonology 1998;26 (Suppl 17):355. [CFGD Register: GN123a; ]
-
- Bucuvalas JC, Chernausek SD, Alfaro MP, Krug SK, Ritschel W, Wilmott RW. Effect of insulin‐like growth factor‐1 treatment in children with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2001;33(5):576‐81. [CFGD Register: GN123b; ] - PubMed
Darmaun 2004 {published data only}
-
- Darmaun D, Hayes V, Schaeffer D, Welch S, Mauras N. Effects of glutamine and recombinant human growth hormone on protein metabolism in prepubertal children with cystic fibrosis. Journal of Clinical Endocrinology and Metabolism 2004;89(3):1146‐52. [CFGD Register: GN90b] - PubMed
-
- Schaeffer D, Darmaun D, Punati J, Mauras N, Hayes VY. Use of glutamine and recombinant human growth hormone (RHGH) in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2000;30 (Suppl 20):323. [CFGD Register: GN90a]
Eubanks 2002 {published data only}
-
- Eubanks V, Atchison J, Arani R, Clancy JP, Sorscher EJ, Wooldridge N, et al. Effects of megestrol acetate on energy intake, weight gain, body composition and resting energy expenditure in cystic fibrosis patients [abstract]. Pediatric Pulmonology 2000; Vol. 30 (Suppl 20):322‐3. [CFGD Register: GN86a; ]
-
- Eubanks V, Koppersmith N, Wooldridge N, Clancy JP, Lyrene R, Arani RB, et al. Effects of megestrol acetate on weight gain, body composition, and pulmonary function in patients with cystic fibrosis. Journal of Pediatrics 2002;140(4):439‐44. [CFGD Register: GN86b; ] - PubMed
Ghergherechi 2017 {published data only}
-
- Ghergherechi R, Rafeey M, Habibzadeh A, Zamani M, Ansarin Kh, Sadeghi Shabestari M. Recombinant human growth hormone effects on growth in cystic fibrosis. Medical Journal of Tabriz University of Medical Sciences and Health Services. 2017 October;39(4):71‐77.
Hardin 1997 {published data only}
-
- Hardin DS, Sy JP. Effects of growth hormone treatment in children with cystic fibrosis: the National Cooperative Growth Study experience. Journal of Pediatrics 1997;131(1 Pt 2):S65‐9. [PUBMED: 9255232] - PubMed
Hardin 1998 {published data only}
-
- Hardin DS, Stratton R, Kramer JC, Reyes de la Rocha S, Govaerts K, Wilson DP. Growth hormone improves weight velocity and height velocity in prepubertal children with cystic fibrosis. Hormone and Metabolic Research 1998;30(10):636‐41. [PUBMED: 9851673] - PubMed
Hardin 2005c {published data only}
-
- Hardin DS, Ferkol T, Ahn C, Dreimane D, Dyson M, Morse M, et al. A retrospective study of growth hormone use in adolescents with cystic fibrosis. Clinical Endocrinology 2005;62(5):560‐6. [PUBMED: 15853825] - PubMed
Huseman 1996 {published data only}
-
- Huseman CA, Colombo JL, Brooks MA, Smay JR, Greger NG, Sammut PH, et al. Anabolic effect of biosynthetic growth hormone in cystic fibrosis patients. Pediatric Pulmonology 1996;22(2):90‐5. [PUBMED: 8875581] - PubMed
Kissner 2000 {published data only}
-
- Kissner DG. Role of progestational agents in the treatment of undernourished patients with cystic fibrosis [letter]. Pediatric Pulmonology 2000;29(3):244. [CFGD Register: GN93; ] - PubMed
Marchand 2000 {published data only}
-
- Marchand V, Baker SS, Baker RD. Leptin level in children with cystic fibrosis, effect megestrol acetate treatment [abstract]. Journal of Pediatric Gastroenterology and Nutrition 1999;29:512. [CFGD Register: GN94b; ] - PubMed
-
- Marchand V, Baker SS, Stark TJ, Baker RD. Randomized, double‐blind, placebo‐controlled pilot trial of megestrol acetate in malnourished children with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2000;31(3):264‐9. [CFGD Register: GN94a; ] - PubMed
NCT00803179 {published data only}
-
- NCT00803179. Growth hormone therapy for wasting in cystic fibrosis. clinicaltrials.gov/ct2/show/NCT00803179 (first received 05 December 2008).
Sackey 1995 {published data only}
-
- Sackey AH, Taylor CJ, Barraclough M, Wales J, Pickering M. Growth hormone as a nutritional adjunct in cystic fibrosis: results of a pilot study. Journal of Human Nutrition 1995;8(3):185‐91.
Safai‐Kutti 1991 {published data only}
-
- Safai‐Kutti S, Selin E, Larsson S, Jagenburg R, Denfors I, Sten G, et al. Zinc therapy in children with cystic fibrosis. Beitrage Zur Infusiontherapie 1991;27:104‐14. [CFGD Register: GN36; ] - PubMed
Vanderwel 2006 {published data only}
-
- Vanderwel M, Hardin DS. Growth hormone normalizes pubertal onset in children with cystic fibrosis. Journal of Pediatric Endocrinology and Metabolism 2006;19(3):237‐44. [PUBMED: 16607924] - PubMed
Additional references
ACSM 2006
-
- American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 7th Edition. Baltimore: Lippincott, Williams, & Wilkins, 2006.
Assael 2009
-
- Assael BM, Casazza G, Iansa P, Volpi S, Milani S. Growth and long‐term lung function in cystic fibrosis: a longitudinal study of patients diagnosed by neonatal screening. Pediatric Pulmonology 2009;44(3):209‐15. [PUBMED: 19230003] - PubMed
Barkhouse 1989
-
- Barkhouse LB, Fahey J, Gillespie CT, Cole DE. Quantitating the effect of cystic fibrosis on linear growth by mathematical modelling of longitudinal growth curves. Growth, Development, and Ageing 1989;53(4):185‐90. [PUBMED: 2638347] - PubMed
Beker 2001
-
- Beker LT, Russek‐Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. Journal of the American Dietetic Association 2001;101(4):438‐42. - PubMed
Borowitz 2002
-
- Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2002;35(3):246‐59. - PubMed
Bryant 2002
-
- Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al. Clinical effectiveness and cost‐effectiveness of growth hormone in children: a systematic review and economic evaluation. Health Technology Assessment 2002;6(18):1‐168. - PubMed
Byard 1994
-
- Byard PJ. The adolescent growth spurt in children with cystic fibrosis. Annals of Human Biology 1994;21(3):229‐40. - PubMed
CFF 2016
-
- Cystic Fibrosis Foundation, Bethesda, Maryland. Cystic Fibrosis Foundation Patient Registry. Annual Data Report 2016:https://www.cff.org/Research/Researcher‐Resources/Patient‐Registry/2016‐....
Coffey 2017
-
- Coffey MJ, Whitaker V, Gentin N, Junek R, Shalhoub C, Nightingale S, et al. Differences in Outcomes between Early and Late Diagnosis of Cystic Fibrosis in the Newborn Screening Era. The Journal of pediatrics 2017;181:137‐145.e1. [PUBMED: 27837951] - PubMed
Corey 1988
-
- Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. Journal of Clinical Epidemiology 1988;41(6):583‐91. [PUBMED: 3260274] - PubMed
Cutfield 2000
-
- Cutfield WS, Wilton P, Bennmarker H, Albertsson‐Wikland K, Chatelain P, Ranke MB, et al. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth‐hormone treatment. Lancet 2000;355(9204):610‐3. [PUBMED: 10696981] - PubMed
Dalzell 1992
-
- Dalzell AM, Shepherd RW, Dean B, Cleghorn GJ, Holt TL, Francis PJ. Nutritional rehabilitation in cystic fibrosis: a 5 year follow‐up study. Journal of Pediatric Gastroenterology and Nutrition 1992;15(2):141‐5. - PubMed
De Benedetti 1997
-
- Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin‐like growth factor‐I. A model for stunted growth in children with chronic inflammation. Journal of Clinical Investigation 1997;99(4):643‐50. [PUBMED: 9045866] - PMC - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DGonbehalfoftheCSMG, editor(s). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org.
Farrell 2001
-
- Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai HC, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long‐term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics 2001;107(1):1‐13. [PUBMED: 11134427] - PubMed
Gee 2000
Giovannucci 2002
-
- Giovannucci E, Haiman CA, Platz EA, Hankinson SE, Pollak MN, Hunter DJ. Dinucleotide repeat in the insulin‐like growth factor‐I gene is not related to risk of colorectal adenoma. Cancer Epidemiology, Biomarkers and Prevention 2002;11(11):1509‐10. - PubMed
Haeusler 1994
-
- Haeusler G, Frisch H, Waldhor T, Gotz M. Perspectives of longitudinal growth in cystic fibrosis from birth to adult age. European Journal of Pediatrics 1994;153(3):158‐63. - PubMed
Hardin 2004
-
- Hardin DS. GH improves growth and clinical status in children with cystic fibrosis ‐‐ a review of published studies. European Journal of Endocrinology / European Federation of Endocrine Societies 2004;151(Suppl 1):S81‐5. - PubMed
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC on behalf of the CSMG and the CBMG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG on behalf of the CSMG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jeffcoate 2002
-
- Jeffcoate W. Growth hormone therapy and its relationship to insulin resistance, glucose intolerance and diabetes mellitus: a review of recent evidence. Drug Safety 2002;25(3):199‐212. - PubMed
Karlberg 1991
-
- Karlberg J, Kjellmer I, Kristiansson B. Linear growth in children with cystic fibrosis. I. Birth to 8 years of age. Acta Paediatrica Scandinavica 1991;80(5):508‐14. [PUBMED: 1872173] - PubMed
Kawchak 1996
-
- Kawchak DA, Zhao H, Scanlin TF, Tomezsko JL, Cnaan A, Stallings VA. Longitudinal, prospective analysis of dietary intake in children with cystic fibrosis. Journal of Pediatrics 1996;129(1):119‐29. [PUBMED: 8757571] - PubMed
Konstan 2003
-
- Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. Journal of Pediatrics 2003;142(6):624‐30. - PubMed
Lai 1998
-
- Lai HC, Kosorok MR, Sondel SA, Chen ST, FitzSimmons SC, Green CG, et al. Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: evaluation of various criteria used to identify malnutrition. Journal of Pediatrics 1998;132(3 Pt 1):478‐85. [PUBMED: 9544905] - PubMed
Laursen 1999
-
- Laursen EM, Lanng S, Rasmussen MH, Koch C, Skakkebaek NE, Muller J. Normal spontaneous and stimulated GH levels despite decreased IGF‐I concentrations in cystic fibrosis patients. European Journal of Endocrinology / European Federation of Endocrine Societies 1999;140(4):315‐21. [PUBMED: 10097250] - PubMed
Leung 2017
Lucidi 2009
-
- Lucidi V, Alghisi F, Raia V, Russo B, Valmarana L, Valmarana R, et al. Growth assessment of paediatric patients with CF comparing different auxologic indicators: A multicentre Italian study. Journal of Pediatric Gastroenterology and Nutrition 2009;49(3):335‐42. [PUBMED: 19543116] - PubMed
Mauch 2016
-
- Mauch RM, Kmit AH, Marson FA, Levy CE, Barros‐Filho AA, Ribeiro JD. Association of growth and nutritional parameters with pulmonary function in cystic fibrosis: a literature review [Associacao dos parametros de crescimento e nutricionais com funcao pulmonar na fibrose cistica: revisao da literatura.]. Revista paulista de pediatria : orgao oficial da Sociedade de Pediatria de Sao Paulo 2016;34(4):503‐9. [PUBMED: 27181343] - PMC - PubMed
Morison 1997
O'Rawe 1992
-
- O'Rawe A, McIntosh I, Dodge JA, Brock DJ, Redmond AO, Ward R, et al. Increased energy expenditure in cystic fibrosis is associated with specific mutations. Clinical Science 1992;82(1):71‐6. [PUBMED: 1310920] - PubMed
O'Riordan 2010
-
- O'Riordan SM, Dattani MT, Hindmarsh PC. Cystic fibrosis‐related diabetes in childhood. Hormone Research in Paediatrics 2010;73(1):15‐24. [PUBMED: 20190536] - PubMed
Patel 2003
Phung 2010
-
- Phung OJ, Coleman CI, Baker EL, Scholle JM, Girotto JE, Makanji SS, et al. Recombinant human growth hormone in the treatment of patients with cystic fibrosis. Pediatrics 2010;126(5):e1211‐26. [PUBMED: 20921071] - PubMed
Quittner 2009
-
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. - PMC - PubMed
Ratjen 2003
-
- Ratjen F, Döring G. Cystic Fibrosis. Lancet 2003;361(9358):681‐9. - PubMed
Reilly 1997
-
- Reilly JJ, Edwards CA, Weaver LT. Malnutrition in children with cystic fibrosis: the energy‐balance equation. Journal of Pediatric Gastroenterology and Nutrition 1997;25(2):127‐36. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen:: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sermet‐Gaudelus 2003
Sharma 2001
Sinaasappel 2002
-
- Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkamp G, Heijerman HG, et al. Nutrition in patients with cystic fibrosis: a European Consensus. Journal of Cystic Fibrosis 2002;1(2):51‐75. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D on behalf of the CBMG, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook forSystematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stettler 2000
-
- Stettler N, Kawchak DA, Boyle LL, Propert KJ, Scanlin TF, Stallings VA, et al. Prospective evaluation of growth, nutritional status, and body composition in children with cystic fibrosis. American Journal of Clinical Nutrition 2000;72(2):407‐13. [PUBMED: 10919935] - PubMed
Tanner 1962
-
- Tanner JM. Growth at Adolescence. Springfield, IL: Charles C Thomas, 1962.
Taylor 1997
Verhelst 2002
-
- Verhelst J, Abs R. Long‐term growth hormone replacement therapy in hypopituitary adults. Drugs 2002;62(16):2399‐412. - PubMed
Wiedemann 2007
-
- Wiedemann B, Paul KD, Stern M, Wagner TO, Hirche TO. Evaluation of body mass index percentiles for assessment of malnutrition in children with cystic fibrosis. European Journal of Clinical Nutrition 2007;61(6):759‐68. [PUBMED: 17213872] - PubMed
Williams 2011
-
- Melmed S, Polonsky K, ReedLarsen P, Kronenberg H. Williams Textbook of Endocrinology. 12th Edition. St. Louis, Missouri, USA: Elsevier, W.B. Saunders, 2011.
Wilson 2003
-
- Wilson TA, Rose SR, Cohen P, Rogol AD, Backeljauw P, Brown R, et al. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. Journal of Pediatrics 2003;143(4):415‐21. [PUBMED: 14571209] - PubMed
Zemel 2000
-
- Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. The Journal of pediatrics 2000;137(3):374‐80. [PUBMED: 10969263] - PubMed
References to other published versions of this review
Thaker 2013
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical